Search
Thermo Scientific Chemicals
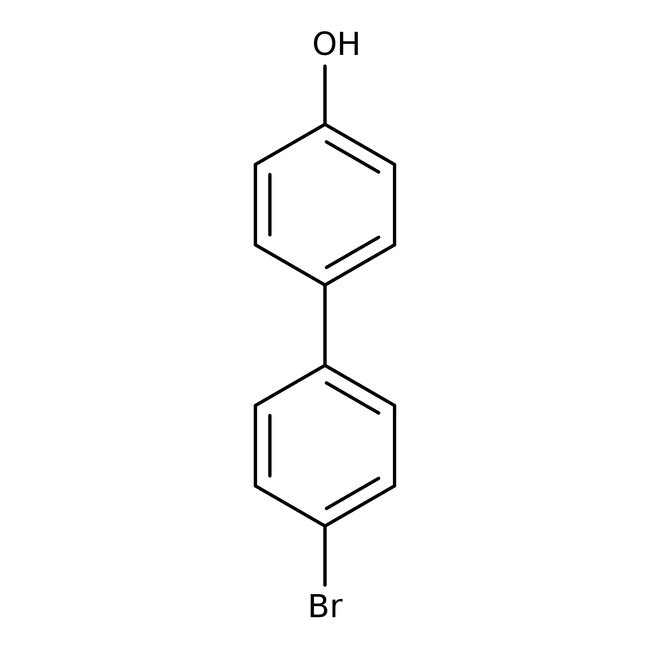
4-Bromo-4'-hydroxybiphenyl, 98%
CAS: 29558-77-8 | C12H9BrO | 249.107 g/mol
Catalog number A10819.06
also known as A10819-06
Price (USD)
55.65
Online Exclusive
61.50Save 5.85 (10%)
Each
Quantity:
5 g
Price (USD)
55.65
Online Exclusive
61.50Save 5.85 (10%)
Each
Chemical Identifiers
CAS29558-77-8
IUPAC Name4'-bromo-[1,1'-biphenyl]-4-ol
Molecular FormulaC12H9BrO
InChI KeyARUBXNBYMCVENE-UHFFFAOYSA-N
SMILESOC1=CC=C(C=C1)C1=CC=C(Br)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream
FormPowder
Assay (Silylated GC)≥97.5%
Melting Point (clear melt)164.0-170.0°C
A biphenyl starting material. 4-Bromo-(1,1-biphenyl)-4-ol is a useful intermediate. Vinylation of 4-bromo-4-hydroxybiphenyl and ethyl acrylate using Pd (OAc) 2/PPh 3 catalyst was studied. Ethyl 4-(4-hydroxyphenyl) cinnamate was formed as the vinylation product, while, 4-hydroxybiphenyl and ethyl cinnamate were formed as side products. Preparation of 4-cyano-4'-hydroxybiphenyl This was prepared from 4-bromo-4'-benzenesulphonyloxybiphenyl by first hydrolysing it to 4-bromo-4-hydroxybiphenyl using sodium hydroxide dissolved in a mixture of water and dioxan. The syntheses of the Nanocomposite dendrimers based on cyclic phosphazene cores: Amorphous materials, were accomplished by following a modified literature procedure by reacting phosphonitrilic chloride trimer with 4-bromophenol or 4-bromo-4-hydroxybiphenyl, respectively, in the presence of K 2 CO 3 in tetrahydrofuran (THF).
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
A biphenyl starting material. 4′-Bromo-(1,1′-biphenyl)-4-ol is a useful intermediate. Vinylation of 4-bromo-4′-hydroxybiphenyl and ethyl acrylate using Pd (OAc) 2/PPh 3 catalyst was studied. Ethyl 4-(4′-hydroxyphenyl) cinnamate was formed as the vinylation product, while, 4-hydroxybiphenyl and ethyl cinnamate were formed as side products. Preparation of 4-cyano-4′-hydroxybiphenyl This was prepared from 4-bromo-4′-benzenesulphonyloxybiphenyl by first hydrolysing it to 4-bromo-4-hydroxybiphenyl using sodium hydroxide dissolved in a mixture of water and dioxan. The syntheses of the Nanocomposite dendrimers based on cyclic phosphazene cores: Amorphous materials, were accomplished by following a modified literature procedure by reacting phosphonitrilic chloride trimer with 4-bromophenol or 4-bromo-4′-hydroxybiphenyl, respectively, in the presence of K 2 CO 3 in tetrahydrofuran (THF).
Solubility
Soluble in water (partly), and methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
A biphenyl starting material. 4′-Bromo-(1,1′-biphenyl)-4-ol is a useful intermediate. Vinylation of 4-bromo-4′-hydroxybiphenyl and ethyl acrylate using Pd (OAc) 2/PPh 3 catalyst was studied. Ethyl 4-(4′-hydroxyphenyl) cinnamate was formed as the vinylation product, while, 4-hydroxybiphenyl and ethyl cinnamate were formed as side products. Preparation of 4-cyano-4′-hydroxybiphenyl This was prepared from 4-bromo-4′-benzenesulphonyloxybiphenyl by first hydrolysing it to 4-bromo-4-hydroxybiphenyl using sodium hydroxide dissolved in a mixture of water and dioxan. The syntheses of the Nanocomposite dendrimers based on cyclic phosphazene cores: Amorphous materials, were accomplished by following a modified literature procedure by reacting phosphonitrilic chloride trimer with 4-bromophenol or 4-bromo-4′-hydroxybiphenyl, respectively, in the presence of K 2 CO 3 in tetrahydrofuran (THF).
Solubility
Soluble in water (partly), and methanol.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents.
RUO – Research Use Only
General References:
- Ashutosh Anant Kelkara,; Taka-aki Hanaoka,; Yoshihiro Kubota,; Yoshihiro Sugi. Palladium catalyzed vinylation of 4-bromo-4'-hydroxybiphenyl. Journal of Molecular Catalysis. 1994, 88 (2) ,L113-L116.
- Sierra Rayne,; Michael G Ikonomou,; MacMurray D Whale. Anaerobic microbial and photochemical degradation of 4,4'-dibromodiphenyl ether. Water Research. 2003, 37(3), 551-560.