Search
Thermo Scientific Chemicals
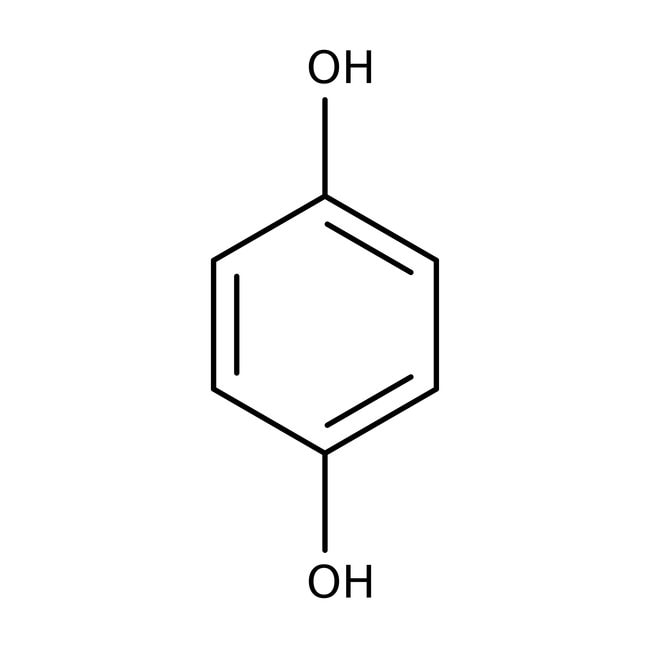
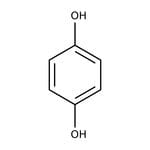
Hydroquinone, 99%
CAS: 123-31-9 | C6H6O2 | 110.112 g/mol
Catalog number A11411.0B
also known as A11411-0B
Price (USD)
84.20
Each
Quantity:
1000 g
Price (USD)
84.20
Each
Chemical Identifiers
CAS123-31-9
IUPAC Namebenzene-1,4-diol
Molecular FormulaC6H6O2
InChI KeyQIGBRXMKCJKVMJ-UHFFFAOYSA-N
SMILESOC1=CC=C(O)C=C1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to pale cream
FormCrystals or powder or crystalline powder
Assay (GC)≥98.5%
Melting Point (clear melt)170.0-176.0?C
Identification (FTIR)Conforms
Hydroquinone is used as a reducing agent and finds applications in black and white photographic developers for film and paper along with 4-(methylamino)phenol sulfate (metol). In this application, silver halides are reduced to elemental silver. It acts as a polymerization inhibitor which prevents polymerization of acrylic acid, methyl methacrylate, cyanoacrylate and other monomers. It undergoes mild oxidation to convert into parabenzoquinone. It is also used as an intermediate to produce antioxidants for rubber and food.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Hydroquinone is used as a reducing agent and finds applications in black and white photographic developers for film and paper along with 4-(methylamino)phenol sulfate (metol). In this application, silver halides are reduced to elemental silver. It acts as a polymerization inhibitor which prevents polymerization of acrylic acid, methyl methacrylate, cyanoacrylate and other monomers. It undergoes mild oxidation to convert into parabenzoquinone. It is also used as an intermediate to produce antioxidants for rubber and food.
Solubility
Soluble in water, methanol, ethanol, diethyl ether, acetone, benzene and carbon tetrachloride.
Notes
Air and light sensitive. Incompatible with strong bases and strong oxidizing agents.
Hydroquinone is used as a reducing agent and finds applications in black and white photographic developers for film and paper along with 4-(methylamino)phenol sulfate (metol). In this application, silver halides are reduced to elemental silver. It acts as a polymerization inhibitor which prevents polymerization of acrylic acid, methyl methacrylate, cyanoacrylate and other monomers. It undergoes mild oxidation to convert into parabenzoquinone. It is also used as an intermediate to produce antioxidants for rubber and food.
Solubility
Soluble in water, methanol, ethanol, diethyl ether, acetone, benzene and carbon tetrachloride.
Notes
Air and light sensitive. Incompatible with strong bases and strong oxidizing agents.
RUO – Research Use Only
General References:
- Widely used as a radical trap to inhibit polymerization and peroxidation reactions.
- Forms inclusion compounds (clathrates) with three molecules of hydroquinone held together by H bonds to form a cage in which a single guest molecule can be accommodated. For a review, see: D. D. MacNicol in Inclusion Compounds, Vol. 2, J. L. Atwood et al, Eds., Academic Press, London (1984), p1.
- A stable 1:1 complex with hydrazine has been isolated and found to be a useful, safer replacement for anhydrous hydrazine, e.g. in the solid state reaction with esters to give hydrazides: J. Chem. Soc., Chem. Commun., 1531 (1995).
- Boota, M.; Hatzell, K. B.; Kumbur, E. C.; Gogotsi, Y. Towards High-Energy-Density Pseudocapacitive Flowable Electrodes by the Incorporation of Hydroquinone. ChemSusChem 2015, 8 (5), 835-843.
- Buzzo, G. S.; Rodrigues, A. C. B.; De Souza, R. F. B.; Silva, J. C. M.; Bastos, E. L.; Spinace, E. V.; Assumpcao, M. H. M. T. Synthesis of hydroquinone with co-generation of electricity from phenol aqueous solution in a proton exchange membrane fuel cell reactor. Catal. Commun. 2015, 59, 113-115.