Search
Thermo Scientific Chemicals
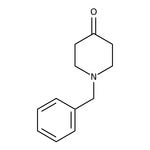
1-Benzyl-4-piperidone, 98+%
CAS: 3612-20-2 | C12H15NO | 189.258 g/mol
Catalog number A12390.22
also known as A12390-22
Price (USD)
99.65
Online Exclusive
111.00Save 11.35 (10%)
Each
Quantity:
100 g
Price (USD)
99.65
Online Exclusive
111.00Save 11.35 (10%)
Each
Chemical Identifiers
CAS3612-20-2
IUPAC Name1-benzylpiperidin-4-one
Molecular FormulaC12H15NO
InChI KeySJZKULRDWHPHGG-UHFFFAOYSA-N
SMILESO=C1CCN(CC2=CC=CC=C2)CC1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear colorless to yellow
Assay (GC)≥98.0% (non-U.S. specification)
Assay from Suppliers CofA≥98.0% (U.S. specification)
CommentSpecification differs for U.S. and non-U.S. material where indicated
FormLiquid
View more
1-Benzyl-4-piperidone is a pharmaceutical intermediate, it is used in penfluridol synthesis of bulk drugs.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Benzyl-4-piperidone is a pharmaceutical intermediate, it is used in penfluridol synthesis of bulk drugs.
Solubility
Soluble in water (12 g/100ml at 20°C).
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents.
1-Benzyl-4-piperidone is a pharmaceutical intermediate, it is used in penfluridol synthesis of bulk drugs.
Solubility
Soluble in water (12 g/100ml at 20°C).
Notes
Keep container tightly sealed. Store in cool, dry conditions in well sealed containers. Incompatible with oxidizing agents.
RUO – Research Use Only
General References:
- Christie Morrill.; Neelakandha S. Mani. Synthesis of 4-Arylpiperidines from 1-Benzyl-4-piperidone: Application of the Shapiro Reaction and Alkenylsilane Cross-Coupling. Org. Lett. 2007, 9 (8), 1505-1508.
- Chunmei Gao.; Yuyang Jiang.; Chunyan Tan.; Xuyu Zu.; Huachen Liu.; Derong Cao. Synthesis and potent antileukemic activities of 10-benzyl-9(10H)-acridinones. Bioorganic & Medicinal Chemistry. 2008, 16 (18), 8670-8675.