Search
Thermo Scientific Chemicals
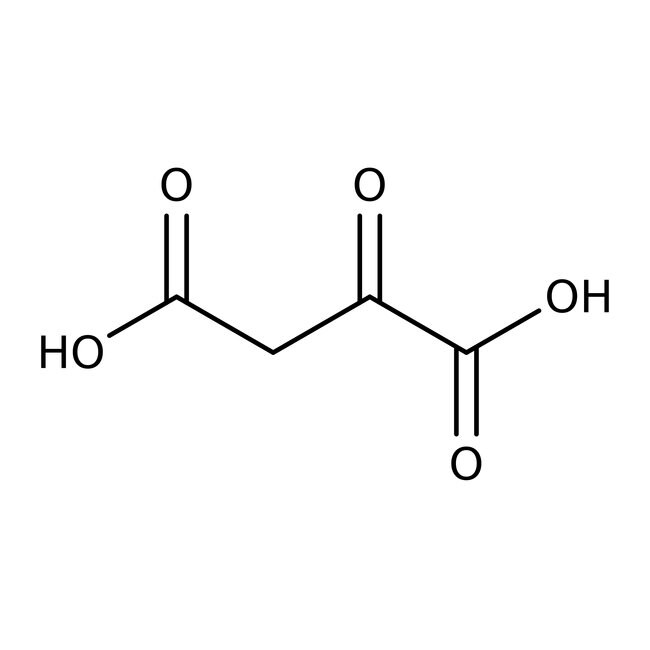
Oxalacetic acid, 97%
CAS: 328-42-7 | C4H4O5 | 132.071 g/mol
Catalog number A12739.06
also known as A12739-06
Price (USD)
97.65
Online Exclusive
108.00Save 10.35 (10%)
Each
Quantity:
5 g
Price (USD)
97.65
Online Exclusive
108.00Save 10.35 (10%)
Each
Chemical Identifiers
CAS328-42-7
IUPAC Name2-oxobutanedioic acid
Molecular FormulaC4H4O5
InChI KeyKHPXUQMNIQBQEV-UHFFFAOYSA-N
SMILESOC(=O)CC(=O)C(O)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream
Assay (Aqueous acid-base Titration)≥96.0 to ≤104.0%
Identification (FTIR)Conforms
FormCrystals or powder or crystalline powder
Oxalacetic acid is used as a metabolic intermediate in many processes that occur in animals and citric acid cycle. It is a used to converted to aspartic acid by aspartate transaminase.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Oxalacetic acid is used as a metabolic intermediate in many processes that occur in animals and citric acid cycle. It is a used to converted to aspartic acid by aspartate transaminase.
Solubility
Soluble in water (0.1 g/ml), ether, ethanol, acetone, and acetone ethyl acetate.
Notes
Store in cool dry place in tightly closed container. With good ventilation. Store away from heat and sunlight.
Oxalacetic acid is used as a metabolic intermediate in many processes that occur in animals and citric acid cycle. It is a used to converted to aspartic acid by aspartate transaminase.
Solubility
Soluble in water (0.1 g/ml), ether, ethanol, acetone, and acetone ethyl acetate.
Notes
Store in cool dry place in tightly closed container. With good ventilation. Store away from heat and sunlight.
RUO – Research Use Only
General References:
- Isamu Suzuki; C.H. Werkman. Chemoautotrophic carbon dioxide fixation by extracts of Thiobacillus thiooxidans. I. Formation of oxalacetic acid. Archives of Biochemistry and Biophysics. 1958, 76(1), 103-111.
- JF Speck. The effect of cations on the decarboxylation of oxalacetic acid. The Journal of Biological Chemistry. 1949, 178315-324.
- Substrate for citrate synthase.