Search
Thermo Scientific Chemicals
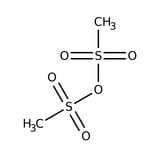
Methanesulfonic anhydride, 97%
CAS: 7143-01-3 | C2H6O5S2 | 174.19 g/mol
Catalog number A13328.30
also known as A13328-30
Price (USD)
621.65
Special offer
Online exclusive
Ends: 31-Dec-2025
731.00Save 109.35 (15%)
Each
Quantity:
250 g
Price (USD)
621.65
Special offer
Online exclusive
Ends: 31-Dec-2025
731.00Save 109.35 (15%)
Each
Chemical Identifiers
CAS7143-01-3
IUPAC Namemethanesulfonyl methanesulfonate
Molecular FormulaC2H6O5S2
InChI KeyIZDROVVXIHRYMH-UHFFFAOYSA-N
SMILESCS(=O)(=O)OS(C)(=O)=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to grey to brown
Identification (FTIR)Conforms
FormPowder or crystals and/or chunks
Assay (Titration ex Anhydride)≥96.0 to ≤104.0%
Melting Point (clear melt)58-72°C
Methanesulfonic anhydride is used to prepare methylsulfonyl esters called as mesylates. It finds application in the reaction of aromatic alkylation. It reacts with dimethylsulfoxide to get sulfoxonium complex, which is a useful oxidizing agent.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Methanesulfonic anhydride is used to prepare methylsulfonyl esters called as mesylates. It finds application in the reaction of aromatic alkylation. It reacts with dimethylsulfoxide to get sulfoxonium complex, which is a useful oxidizing agent.
Solubility
Soluble in organic solvents.
Notes
Moisture sensitive. Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
Methanesulfonic anhydride is used to prepare methylsulfonyl esters called as mesylates. It finds application in the reaction of aromatic alkylation. It reacts with dimethylsulfoxide to get sulfoxonium complex, which is a useful oxidizing agent.
Solubility
Soluble in organic solvents.
Notes
Moisture sensitive. Hygroscopic. Keep the container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- More powerful sulfonylating reagent than the sulfonyl chloride, also avoiding possible displacement of sulfonate by halide. With a catalytic amount of the acid effects mesylation of deactivated aromatics such as 1,3-dichlorobenzene: Chem. Lett., 395 (1988); mesyl chloride is ineffective.
- Primary and secondary alcohols may be oxidized to carbonyl compounds by sulfonic anhydrides in DMSO: J. Org. Chem., 39, 1977 (1974). For related methods, see Dimethyl sulfoxide, A13280.
- Liu, W., Tilley, T. D. Sterically Controlled Functionalization of Carbon Surfaces with- C6H4CH2X (X= OSO2Me or N3) Groups for Surface Attachment of Redox-Active Molecules. Langmuir 2015, 31 (3), 1189-1195.
- Hu, Y.; Zhang, F.; Zhang, C.; Zhang, M. Anti-inflammatory Properties of an Active Sesquiterpene Lactone and its Structure-Activity Rela-tionship. Med chem 2015, 5, 354-360.