Search
Thermo Scientific Chemicals
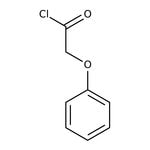
Phenoxyacetyl chloride, 98%
CAS: 701-99-5 | C8H7ClO2 | 170.59 g/mol
Catalog number A13761.30
also known as A13761-30
Price (USD)
196.65
Online Exclusive
218.00Save 21.35 (10%)
Each
Quantity:
250 g
Price (USD)
196.65
Online Exclusive
218.00Save 21.35 (10%)
Each
Chemical Identifiers
CAS701-99-5
IUPAC Name2-phenoxyacetyl chloride
Molecular FormulaC8H7ClO2
InChI KeyPKUPAJQAJXVUEK-UHFFFAOYSA-N
SMILESClC(=O)COC1=CC=CC=C1
View more
Specifications Specification Sheet
Specification Sheet
Assay (Titration ex Chloride)≥97.5%
Refractive Index1.5320-1.5360 @ 20?C
FormLiquid
Identification (FTIR)Conforms
Appearance (Color)Clear colorless to yellow to brown
Acylation of D- and DL-WV with phenoxyacetyl chloride, followed by cleavage of the t-butyl ester,afforded the penultimate penicilloic acids. Phenoxyacetyl chloride was used in the synthesis of series of macrocyclic bis-β-lactams, 5-phenyl-6-epiphenoxymethylpenicillin benzyl ester, N-protected guanosine derivatives, useful in RNA synthesis, phenyloxyketene, for cycloaddition to imines leading to β-lactams. Treatment of iminophosphoranes with phenoxyacetyl chloride afforded respectively the trans- and cis-3-(acylamino)-p-lactams. By the oxidative addition of stannous fluoride to allyliodide, with 1, 2-O-isopropylidene-D-glyceraldehyde and phenoxyacetyl chloride results in the predominant formation of erythro homoallylester, which is in turn converted into 2-deoxy-D-ribose. The 4-disubstituted P-lactams (4a-c and 6a-b) were prepared through the reaction of N,N-diphenylhydrazones and N-methyl-N-phenylhydrazones of ketones with phenoxyacetyl chloride/Et3N in dichloromethane.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Acylation of D- and DL-WV with phenoxyacetyl chloride, followed by cleavage of the t-butyl ester,afforded the penultimate penicilloic acids. Phenoxyacetyl chloride was used in the synthesis of series of macrocyclic bis-β-lactams, 5-phenyl-6-epiphenoxymethylpenicillin benzyl ester, N-protected guanosine derivatives, useful in RNA synthesis, phenyloxyketene, for cycloaddition to imines leading to β-lactams. Treatment of iminophosphoranes with phenoxyacetyl chloride afforded respectively the trans- and cis-3-(acylamino)-p-lactams. By the oxidative addition of stannous fluoride to allyliodide, with 1, 2-O-isopropylidene-D-glyceraldehyde and phenoxyacetyl chloride results in the predominant formation of erythro homoallylester, which is in turn converted into 2-deoxy-D-ribose. The 4-disubstituted P-lactams (4a-c and 6a-b) were prepared through the reaction of N,N-diphenylhydrazones and N-methyl-N-phenylhydrazones of ketones with phenoxyacetyl chloride/Et3N in dichloromethane.
Notes
Moisture Sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents, moisture.
Acylation of D- and DL-WV with phenoxyacetyl chloride, followed by cleavage of the t-butyl ester,afforded the penultimate penicilloic acids. Phenoxyacetyl chloride was used in the synthesis of series of macrocyclic bis-β-lactams, 5-phenyl-6-epiphenoxymethylpenicillin benzyl ester, N-protected guanosine derivatives, useful in RNA synthesis, phenyloxyketene, for cycloaddition to imines leading to β-lactams. Treatment of iminophosphoranes with phenoxyacetyl chloride afforded respectively the trans- and cis-3-(acylamino)-p-lactams. By the oxidative addition of stannous fluoride to allyliodide, with 1, 2-O-isopropylidene-D-glyceraldehyde and phenoxyacetyl chloride results in the predominant formation of erythro homoallylester, which is in turn converted into 2-deoxy-D-ribose. The 4-disubstituted P-lactams (4a-c and 6a-b) were prepared through the reaction of N,N-diphenylhydrazones and N-methyl-N-phenylhydrazones of ketones with phenoxyacetyl chloride/Et3N in dichloromethane.
Notes
Moisture Sensitive. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Store away from strong oxidizing agents, moisture.
RUO – Research Use Only
General References:
- Ajay K. Bose,; J. C. Kapur,; J. L. Fahey,; M. S. Manhas. Lactams. XXIX. Synthesis of aza analogs of cepham . J. Org. Chem., . 1973, 38(19), 3437-3438.
- John C. Sheehan,; Kenneth R. Henery-Logan. The Total Synthesis of Penicillin V. J. Am. Chem. Soc., 1959, 81(12), 3089-3094.
- Can be used to protect OH groups. The phenoxyacetate group is 50x more labile to aqueous ammonia than acetate: Tetrahedron Lett., 4273 (1968). It can also be cleaved with methanolic t-BuNH 2 : Chem. Lett., 965 (1982).