Search
Thermo Scientific Chemicals
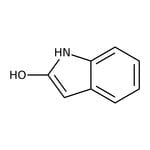
Oxindole, 97+%
CAS: 59-48-3 | C8H7NO | 133.15 g/mol
Catalog number A18764.22
also known as A18764-22
Price (USD)
434.65
Online Exclusive
483.00Save 48.35 (10%)
Each
Quantity:
100 g
Price (USD)
434.65
Online Exclusive
483.00Save 48.35 (10%)
Each
Chemical Identifiers
CAS59-48-3
IUPAC Name1H-indol-2-ol
Molecular FormulaC8H7NO
InChI KeyJHFAEUICJHBVHB-UHFFFAOYSA-N
SMILESOC1=CC2=CC=CC=C2N1
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to dark cream to cream to brown
Identification (FTIR)Conforms
FormPowder
Melting Point (clear melt)120.0-130.0?C
Assay (GC)≥97.0%
Oxindole is considered as indole analogue, which shows pharmacological activity.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Oxindole is considered as indole analogue, which shows pharmacological activity.
Solubility
Soluble in methanol, ethanol, dimethyl sulfoxide and ether. Insoluble in water.
Notes
Incompatible with strong oxidizing agents.
Oxindole is considered as indole analogue, which shows pharmacological activity.
Solubility
Soluble in methanol, ethanol, dimethyl sulfoxide and ether. Insoluble in water.
Notes
Incompatible with strong oxidizing agents.
RUO – Research Use Only
General References:
- Yang, H. B.; Zhao, Y. Z.; Sang, R.; Shi, M. (DHQ)2AQN-Catalyzed Asymmetric Substitution of Isatin-Derived Hydrazones with O-Boc-Protected Morita-Baylis-Hillman Adducts: A Strategy for Synthesizing Enantioenriched Azo Compounds Incorporating an Oxindole Scaffold. J. Org. Chem. 2014, 79 (8), 3519-3528.
- Hinze, M. E.; Daughtry, J. L.; Lewis, C. A. Access to the Surugatoxin Alkaloids: Chemo-, Regio-, and Stereoselective Oxindole Annulation. J. Org. Chem. 2015, 80 (22), 11258-11265.