Search
Thermo Scientific Chemicals
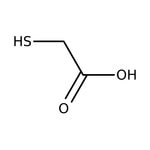
Mercaptoacetic acid, 97+%
Air Senstitive | CAS: 68-11-1 | C2H4O2S | 92.112 g/mol
Catalog number B20391.22
also known as B20391-22
Price (USD)
37.90
Each
Quantity:
100 g
Price (USD)
37.90
Each
Chemical Identifiers
CAS68-11-1
IUPAC Name2-sulfanylacetic acid
Molecular FormulaC2H4O2S
InChI KeyCWERGRDVMFNCDR-UHFFFAOYSA-N
SMILESOC(=O)CS
View more
Specifications Specification Sheet
Specification Sheet
CommentMaterial sourced in the U.S. and in other countries
Identification (FTIR)Conforms (non-U.S. sourced material)
Proton NMRConforms to structure (non-U.S. sourced material)
Refractive Index1.5020-1.5070 @ 20°C (non-U.S. sourced material)
Assay (Aqueous acid-base Titration)≥97.0 to ≤103.0% (non-U.S. sourced material)
View more
Reagent that protects tryptophan in amino acid analysis.Mercaptoacetic acid is used as a protecting agent for tryptophan in amino acid analysis and an acidity indicator. It finds application as an intermediate in the chemical reactions such as addition, elimination and cyclization. It acts as a precursor to ammonium thioglycolate, sodium thioglycolate and calcium thioglycolate. Its organotin derivatives are used as stabilizers for polyvinyl chloride (PVC). In organic synthesis, it acts as a nucleophile in thioglycolysis reactions and sulfur transfer agent for sulfonyl chloride synthesis. Further, it is used in leather processing.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Reagent that protects tryptophan in amino acid analysis.Mercaptoacetic acid is used as a protecting agent for tryptophan in amino acid analysis and an acidity indicator. It finds application as an intermediate in the chemical reactions such as addition, elimination and cyclization. It acts as a precursor to ammonium thioglycolate, sodium thioglycolate and calcium thioglycolate. Its organotin derivatives are used as stabilizers for polyvinyl chloride (PVC). In organic synthesis, it acts as a nucleophile in thioglycolysis reactions and sulfur transfer agent for sulfonyl chloride synthesis. Further, it is used in leather processing.
Solubility
Miscible with water, ethanol, ethers, ketones, esters, chlorinated hydrocarbons, benzene and aromatic hydrocarbons. Slightly miscible with chloroform.
Notes
Air sensitive. Incompatible with strong oxidizing agents and strong acids.
Reagent that protects tryptophan in amino acid analysis.Mercaptoacetic acid is used as a protecting agent for tryptophan in amino acid analysis and an acidity indicator. It finds application as an intermediate in the chemical reactions such as addition, elimination and cyclization. It acts as a precursor to ammonium thioglycolate, sodium thioglycolate and calcium thioglycolate. Its organotin derivatives are used as stabilizers for polyvinyl chloride (PVC). In organic synthesis, it acts as a nucleophile in thioglycolysis reactions and sulfur transfer agent for sulfonyl chloride synthesis. Further, it is used in leather processing.
Solubility
Miscible with water, ethanol, ethers, ketones, esters, chlorinated hydrocarbons, benzene and aromatic hydrocarbons. Slightly miscible with chloroform.
Notes
Air sensitive. Incompatible with strong oxidizing agents and strong acids.
RUO – Research Use Only
General References:
- Si, L.; Ariya, P. A. Photochemical reactions of divalent mercury with thioglycolic acid: Formation of mercuric sulfide particles. Chemosphere 2015, 119, 467-472.
- Maltas, E.; Ertekin, B. Binding of actin to thioglycolic acid modified superparamagnetic nanoparticles for antibody conjugation. Int. J. Biol. Macromol. 2015, 72, 984-989.