Search
Thermo Scientific Chemicals
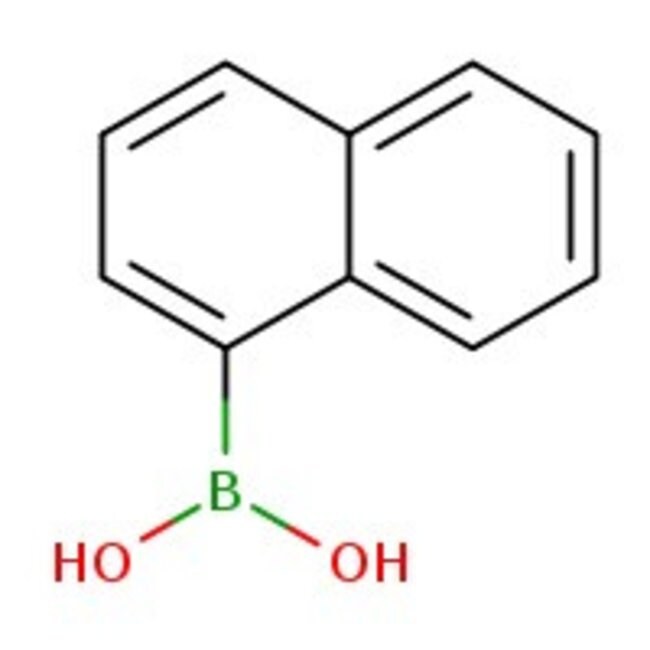
1-Naphthaleneboronic acid, 97%
CAS: 13922-41-3 | C10H9BO2 | 171.99 g/mol
Catalog number B21219.03
also known as B21219-03
Price (USD)
35.65
Online Exclusive
39.70Save 4.05 (10%)
Each
Quantity:
1 g
Price (USD)
35.65
Online Exclusive
39.70Save 4.05 (10%)
Each
Chemical Identifiers
CAS13922-41-3
IUPAC Name(naphthalen-1-yl)boronic acid
Molecular FormulaC10H9BO2
InChI KeyHUMMCEUVDBVXTQ-UHFFFAOYSA-N
SMILESOB(O)C1=CC=CC2=CC=CC=C12
View more
Specifications Specification Sheet
Specification Sheet
Proton NMRConforms to structure
Appearance (Color)White to pale cream
FormCrystals or powder or crystalline powder
Assay (Aqueous acid-base Titration)≥96.0%
Assay (HPLC)≥96.0%
1-Naphthaleneboronic acid is a very useful building block for introduction of a naphthyl group through cross-coupling protocols. It is useful in the synthesis of polyaromatic hydrocarbons utilizing the Suzuki reaction.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
1-Naphthaleneboronic acid is a very useful building block for introduction of a naphthyl group through cross-coupling protocols. It is useful in the synthesis of polyaromatic hydrocarbons utilizing the Suzuki reaction.
Solubility
Soluble in methanol. Insoluble in water.
Notes
Incompatible with oxidizing agents. Store in a cool, dry condition in a well sealed container. Store at room temperature.
1-Naphthaleneboronic acid is a very useful building block for introduction of a naphthyl group through cross-coupling protocols. It is useful in the synthesis of polyaromatic hydrocarbons utilizing the Suzuki reaction.
Solubility
Soluble in methanol. Insoluble in water.
Notes
Incompatible with oxidizing agents. Store in a cool, dry condition in a well sealed container. Store at room temperature.
RUO – Research Use Only
General References:
- Cheng-Hsien Yang; Chia-Cheng Tai; Yu-Ting Huang; I-Wen Sun. Ionic liquid promoted palladium-catalyzed Suzuki cross-couplings of N-contained heterocyclic chlorides with naphthaleneboronic acids. Tetrahedron.2005, 61 4857-4861.
- R. L. Letsinger; J. Malcolm Smith; J. Gilpin; D. B. MacLean. Organoboron Compounds. XX. Chemistry of Some 1-Naphthaleneboronic Acids with Substituents in the 8-Position. J. Org. Chem.1965, 30 (3), 807-812.
- The Suzuki coupling with a protected L-tyrosine triflate, catalyzed by Tetrakis(triphenyl phosphine) palladium(0) , 10548, occurred in high yield without racemization when anhydrous K2CO3 in toluene was used as the base: J. Org. Chem., 57, 379 (1992). For Ni-catalyzed coupling with allylamines, see: J. Chem. Soc., Perkin 1, 2083 (1995). See Appendix 5.