Search
Thermo Scientific Chemicals
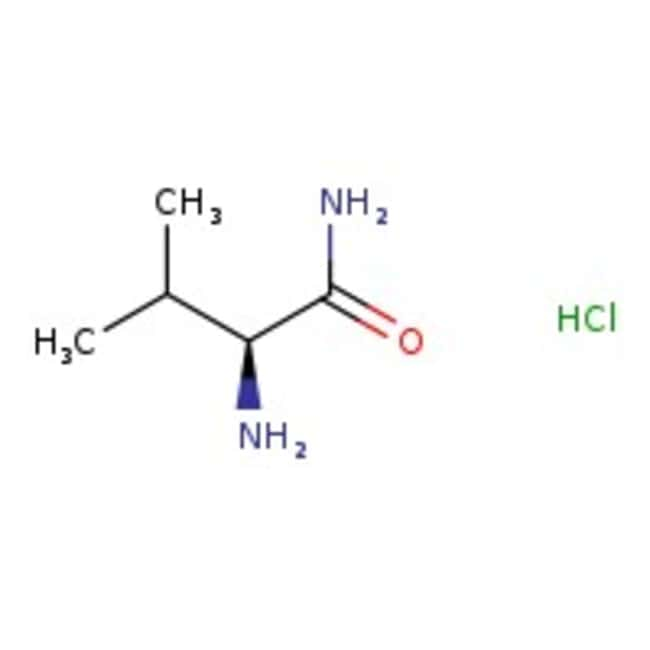
L-Valinamide hydrochloride, 95%
CAS: 3014-80-0 | C5H13ClN2O | 152.62 g/mol
Catalog number H63775.06
also known as H63775-06
Price (USD)
43.70
Each
Quantity:
5 g
Price (USD)
43.70
Each
Chemical Identifiers
CAS3014-80-0
IUPAC Name(2S)-2-amino-3-methylbutanamide hydrochloride
Molecular FormulaC5H13ClN2O
InChI KeyXFCNYSGKNAWXFL-WCCKRBBISA-N
SMILESCl.CC(C)[C@H](N)C(N)=O
View more
Specifications Specification Sheet
Specification Sheet
Assay (HPLC)>94.0%
It is a reagent used in the synthesis of alkylpyrazines. Also used in the synthesis of elastase inhibitory activity
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
It is a reagent used in the synthesis of alkylpyrazines. Also used in the synthesis of elastase inhibitory activity
Solubility
Soluble in methanol (50 mg/ ml-clear, colorless solution).
Notes
Store in cool. Keep container tightly closed in a dry and well-ventilated place. Store away from oxidizing agent.
It is a reagent used in the synthesis of alkylpyrazines. Also used in the synthesis of elastase inhibitory activity
Solubility
Soluble in methanol (50 mg/ ml-clear, colorless solution).
Notes
Store in cool. Keep container tightly closed in a dry and well-ventilated place. Store away from oxidizing agent.
RUO – Research Use Only
General References:
- H.-G. Schmarr; W. Sang; S. Ganß; S. Koschinski; and R. Meusinger.New insights into the synthesis and characterization of 2-methoxy-3-alkylpyrazines and their deuterated isotopologues. Journal of Labelled Compounds and Radiopharmaceuticals. 2011, 54(8), 438-440.
- Rode, H. B.; Sprang, T.; Besch, A.1; Loose, J.; Otto, H.-Hr. Pseudosaccharin amine derivatives: synthesis and elastase inhibitory activity. Die Pharmazie - An International Journal of Pharmaceutical Sciences. 2005, 60(10), 723-731.