Search
Thermo Scientific Chemicals
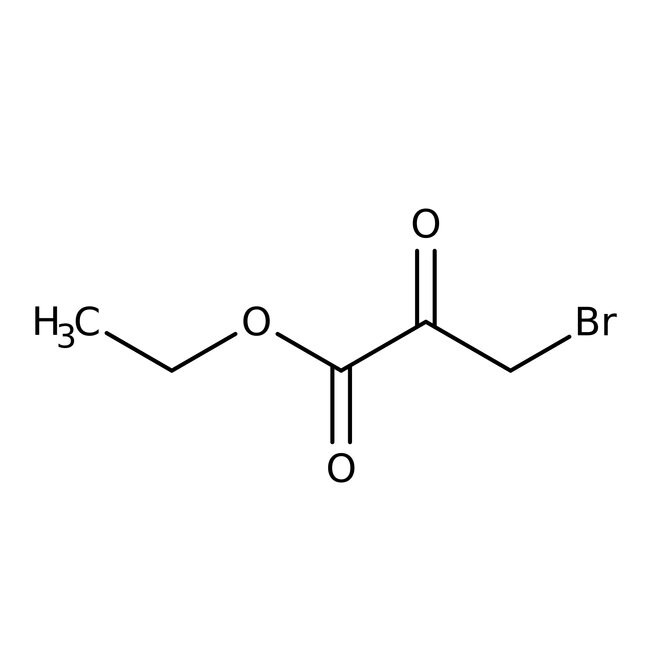
Ethyl 3-bromopyruvate, tech. 75%
CAS: 70-23-5 | C5H7BrO3 | 195.01 g/mol
Catalog number L00582.22
also known as L00582-22
Price (USD)
265.65
Online Exclusive
295.00Save 29.35 (10%)
Each
Quantity:
100 g
Price (USD)
265.65
Online Exclusive
295.00Save 29.35 (10%)
Each
Chemical Identifiers
CAS70-23-5
Specifications Specification Sheet
Specification Sheet
Appearance (Color)Clear yellow to pink-red
Assay (GC)≥70.0%
CommentMay contain HBr gas
Identification (FTIR)Conforms
FormLiquid
Ethyl 3-bromopyruvate is employed in a synthesis of thioxothiazolidines from primary amines and carbon disulfide.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
Ethyl 3-bromopyruvate is employed in a synthesis of thioxothiazolidines from primary amines and carbon disulfide.
Solubility
Difficult to mix.
Notes
Moisture Sensitive & Hygroscopic. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
Ethyl 3-bromopyruvate is employed in a synthesis of thioxothiazolidines from primary amines and carbon disulfide.
Solubility
Difficult to mix.
Notes
Moisture Sensitive & Hygroscopic. Keep container tightly closed. Store in cool, dry conditions in well sealed containers.
RUO – Research Use Only
General References:
- Dale L. Boger.; Chen, J.H. A Modified Friedlander Condensation for the Synthesis of 3-Hydroxyquinoline-2-carboxylates. J. Org. Chem. 1995, 60, (22), 7369-7371.
- Brindaban C. Ranu.; Laksmikanta Adak.; Subhash Banerjee. Ionic liquid promoted interrupted Feist-Benary reaction with high diastereoselectivity. Tetrahedron Letters. 2008, 49, (31), 4613-4617.