Search
Thermo Scientific Chemicals
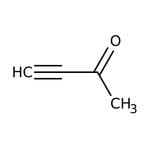
3-Butyn-2-one, 98%
CAS: 1423-60-5 | C4H4O | 68.08 g/mol
Catalog number L05527.03
also known as L05527-03
Price (USD)
70.00
Each
Quantity:
1 g
Price (USD)
70.00
Each
Chemical Identifiers
CAS1423-60-5
Specifications Specification Sheet
Specification Sheet
Refractive Index1.4045-1.4085 @ 20?C
Assay (GC)≥97.5%
Appearance (Color)Clear colorless to yellow
FormLiquid
Identification (FTIR)Conforms
3-Butyn-2-one was used in the synthesis of clerodane diterpenoid (±)-sacacarin. It was used as substrate in stereoselective, conjugate arylation mediated by gallium(III) chloride leading to (E)-α,β-unsaturated ketones. 3-Butyn-2-one undergoes asymmetric double-Michael reaction with ortho-tosylamidophenyl malonate catalyzed by chiral aminophosphines to yield indolines2. It undergoes double Michael reaction with nitrogen-containing tethered diacid to give pipecolic acid derivatives. 3-Butyn-2-one, is used as a reactant with Dimethyl acetone-1,3-dicarboxylate, under mild conditions to give a benzenoid product via a Michael addition - aldol cyclization process.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
3-Butyn-2-one was used in the synthesis of clerodane diterpenoid (+/-)-sacacarin. It was used as substrate in stereoselective, conjugate arylation mediated by gallium(III) chloride leading to (E)-α,β-unsaturated ketones. 3-Butyn-2-one undergoes asymmetric double-Michael reaction with ortho-tosylamidophenyl malonate catalyzed by chiral aminophosphines to yield indolines2. It undergoes double Michael reaction with nitrogen-containing tethered diacid to give pipecolic acid derivatives. 3-Butyn-2-one, is used as a reactant with Dimethyl acetone-1,3-dicarboxylate, under mild conditions to give a benzenoid product via a Michael addition - aldol cyclization process.
Solubility
It is soluble in water.
Notes
Stable under normal temperatures and pressures. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from oxidizing agents.
3-Butyn-2-one was used in the synthesis of clerodane diterpenoid (+/-)-sacacarin. It was used as substrate in stereoselective, conjugate arylation mediated by gallium(III) chloride leading to (E)-α,β-unsaturated ketones. 3-Butyn-2-one undergoes asymmetric double-Michael reaction with ortho-tosylamidophenyl malonate catalyzed by chiral aminophosphines to yield indolines2. It undergoes double Michael reaction with nitrogen-containing tethered diacid to give pipecolic acid derivatives. 3-Butyn-2-one, is used as a reactant with Dimethyl acetone-1,3-dicarboxylate, under mild conditions to give a benzenoid product via a Michael addition - aldol cyclization process.
Solubility
It is soluble in water.
Notes
Stable under normal temperatures and pressures. Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Keep away from oxidizing agents.
RUO – Research Use Only
General References:
- H Monti.; G Audran.; G Léandri.; JP Monti. ZnI 2 Catalyzed [2+ 2] versus [3+ 2] cycloaddition of an allyltrimethylsilane with 3-butyn-2-one: Confirmation of a cyclobutene by-product formation. Tetrahedron letters. 1994, 35,(19), 3073-3076.
- IH Um.; EJ Lee.; JS Min. Remarkable catalytic effect of H+ in Michael-type additions of anilines to 3-butyn-2-one. Tetrahedron letters. 2001, 57,(47), 9585-9589.
- Reacts with Dimethyl acetone-1,3-dicarboxyl ate, A14969, under mild conditions to give a benzenoid product via a Michael addition - aldol cyclization process: Synth. Commun., 28, 1525 (1998):
- For a similar reaction, see Methyl 2-hexynoate, B23065.