Search
Thermo Scientific Chemicals
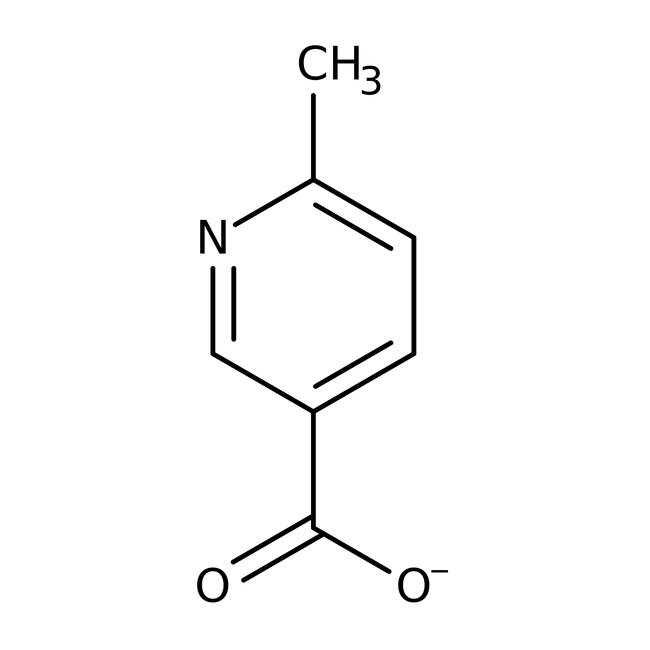
6-Methylnicotinic acid, 99%
CAS: 3222-47-7 | C7H7NO2 | 137.138 g/mol
Catalog number L08877.14
also known as L08877-14
Price (USD)
320.65
Online Exclusive
356.00Save 35.35 (10%)
Each
Quantity:
25 g
Price (USD)
320.65
Online Exclusive
356.00Save 35.35 (10%)
Each
Chemical Identifiers
CAS3222-47-7
IUPAC Name6-methylpyridine-3-carboxylate
Molecular FormulaC7H6NO2
InChI KeyRZOKQIPOABEQAM-UHFFFAOYSA-M
SMILESCC1=CC=C(C=N1)C([O-])=O
View more
Specifications Specification Sheet
Specification Sheet
Appearance (Color)White to cream to pale brown
FormCrystals or powder or crystalline powder or fused solid or flakes or granules
Assay (HPLC)≥98.5%
Assay (Aqueous acid-base Titration)≥98.5 to ≤101.5%
6-Methylnicotinic acid is an intermediate of the drug etoricoxib (a non- steroidal anti-inflammatory drug for the treatment of arthritis and osteoarthritis). It is also used as organic intermediates.
This Thermo Scientific Chemicals brand product was originally part of the Alfa Aesar product portfolio. Some documentation and label information may refer to the legacy brand. The original Alfa Aesar product / item code or SKU reference has not changed as a part of the brand transition to Thermo Scientific Chemicals.
Applications
6-Methylnicotinic acid is an intermediate of the drug etoricoxib (a non- steroidal anti-inflammatory drug for the treatment of arthritis and osteoarthritis). It is also used as organic intermediates.
Solubility
Sparingly soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents. Store away from oxidizing agents.
6-Methylnicotinic acid is an intermediate of the drug etoricoxib (a non- steroidal anti-inflammatory drug for the treatment of arthritis and osteoarthritis). It is also used as organic intermediates.
Solubility
Sparingly soluble in water.
Notes
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Incompatible with strong oxidizing agents. Store away from oxidizing agents.
RUO – Research Use Only
General References:
- Andreas Tinschert; Andreas Kiener; Klaus Heinzmann; A. Tschech. Isolation of new 6-methylnicotinic-acid-degrading bacteria, one of which catalyses the regioselective hydroxylation of nicotinic acid at position C2.Archives of Microbiology.1997, 168 355-361.
- B.-M. Kukovec; Z. Popovic and G. Pavlovic. A new coordination mode of 6-methylnicotinic acid in trans-tetraaquabis(6-methylpyridine-3-carboxylato-[kappa]O)cobalt(II) tetrahydrate.Acta Cryst.2007, C63 m615-m617.
- Dilithiation can be effected with LDA, allowing substitution at the 6-methyl group, as, for example carboxylation in the synthesis of methotrexate analogues: J. Med. Chem., 40, 370 (1997).