Search
High-titer, Flexible Lentiviral RNAi Vector
Interview with Adam Harris
In a recent interview, Adam Harris, an Invitrogen scientist, discussed the advantages of Invitrogen’s new RNAi vector.
Q: What makes Invitrogen’s new HiPerform™ vector an improvement over preceding lentiviral RNAi vectors?
Harris: The HiPerform™ vector contains an mRNA stabilizing sequence (WPRE) and a nuclear import sequence (cPPT), which have generated up to five-fold higher virus titers and EmGFP expression levels in different cell lines (Figure 1). Additionally, Gateway® MultiSite technology allows researchers to express the EmGFP/miR RNAi cassette using the CMV, EF-1a, or their own tissue-specific promoter.
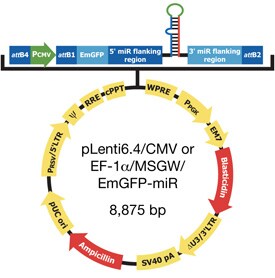
Figure 1A - The new BLOCK-iT™ HiPerform™ Lentiviral POL II miR RNAi Expression System with EmGFP. The pLenti6.4/CMVor Ef-1a/V5-M5--GW/EmGFP-miR vector is driven by the CMV promotor, has the blasticidin resistance marker, and is available with co-cistronic EmGFP expression as a reporter.
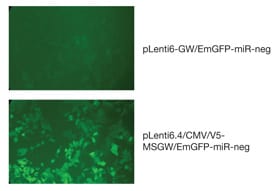
Figure 1B - Higher fluorescence evident from pLenti6.4 constructs using the BLOCK-iT™ HiPerform™ Lentiviral POL II miR RNAi Expression System. Images taken four days following transduction of GripTite™ 293 cells at a MOI of 3 with the respective viruses.
Harris: As with the preceding system, researchers are able to track miRNA expression through co-cistronic expression with EmGFP and knock down more than one gene simultaneously through expression of multiple miRNAs from a single transcript. However, with the new HiPerform™ system another key feature stands out: blasticidin resistance is expressed from the mouse PgK-1 promoter to avoid shutdown after multiple passages.
Q: Is this vector appropriate for in vivo applications?
Harris: That seems to be where the RNAi field is moving because demonstrating phenotypic alterations in animals validates conclusions made in vitro. The MultiSite capability of the new HiPerform™ system makes it simple to insert tissue-specific and other regulated promoters into the system.
Q: Specifically, how are the WPRE and cPPT elements contributing to higher titers?
Harris: cPPT is the central poly purine tract from HIV-1, and this sequence has been shown to be important for import of the provirus into the nucleus. In principal, it has its greatest effects in slowly dividing or non-dividing cells. WPRE is the woodchuck hepatitis virus post-transcriptional regulatory element that has been shown to stabilize and/or improve export of viral transcripts from the nucleus. The WPRE greatly enhances expression of the transcripts in which it is present.
Q: How do you typically measure titers when using the HiPerform™ system?
Harris: We calculate titer with flow cytometry with GFP (infectious or transducing units/ml). Typically target cells are transduced with small volumes of virus such that fewer than 30% of cells are transduced. The transducing units/ml is then calculated based on how many cells score as GFP positive (in most cases due to infection with a single virion) and the volume of viral supernatant applied.
Q: Are you being misled into concluding that titers higher because GFP expression is higher?
Harris: Well, it is true that GFP expression is higher with the HiPerform™ system, but we are able to correlate knock down activity with GFP titer when comparing the preceding system with the new system. This shows that our assessment of titer is not based merely on better detection of GFP in transduced cells.
Q: Briefly, can you describe the HiPerform™ workflow?
Harris: The typical workflow with our new HiPerform™ system and our other lentiviral expression systems includes designing and ordering two 64nt DNA oligos which encode the precursor microRNA. (Editor’s note: These can be conveniently designed and ordered through Invitrogen’s RNAi Designer at www.invitrogen.com/rnaidesigner) These oligos are then annealed and cloned in an efficient five minute ligation step into a pcDNA vector adapted with miRNA flanking regions. The resulting pcDNA vector, which carries the CMV promoter, can be used immediately for transfections to screen for knockdown. One would pick the one or two most potent of the miRNA sequences for going on to use in lentivirus. Once that sequence has been identified, that vector is transferred via a Gateway® recombination reaction along with the CMV, EF-1a, or another promoter of interest into the HiPerform™ Lentiviral destination vector. From the resulting lentiviral expression clone, virus is produced and titered as I described previously. This virus is then used to transduce cells. For short term knockdown it is not necessary to do any kind of selection, but for long term knockdown we select with antibiotics or use FACS to sort out cells which are GFP positive. These cells are then harvested to verify knockdown, by a method such as qPCR or Western blotting. From this we can assess phenotypes that are produced by knocking down that target gene.
Order Now: BLOCK-iT™ HiPerform™ Lentiviral PolII miR RNAi Expression System with EmGFP