Search
Hang Up in the Wells — Preventing Samples from Remaining in the Wells of Your Gels
Washing and Resuspending Pellets
Sample hang-up is usually characterized by radioactive signal at the top of the gel (Figure 1) or aberrant migration of the sample, and it frequently occurs with only 1 or 2 of the samples being analyzed. Most often, this problem can be attributed to a precipitated sample that has not been well washed or resuspended, and the problem can usually be eliminated by taking extra time for sample preparation prior to loading the gel. We suggest that once nucleic acid precipitates have been pelleted by centrifugation, the tube be tilted sideways with the pellet on the top side, and the liquid drawn off from underneath using a Pasteur pipet and manual or vacuum line aspiration. The tube is then centrifuged again for just 3 seconds to collect residual liquid clinging to the walls of the tube. It is important to remove this liquid, since it may contain sufficient salts or dense materials to interfere with normal gel migration. After all liquid has been drawn off, the pellet can be washed by adding 70–80% cold EtOH, vortexing, centrifuging and drawing off the liquid as before. We usually do not dry our pellets with heat or vacuum centrifugation. RNA is especially difficult to resuspend once dry. Allowing the tubes to sit open on the bench top for a few minutes is usually sufficient to permit any remaining EtOH to evaporate. Gel loading buffer is then added to resuspend the pellets. Using the largest volume possible (that will fit into the wells of the gel) will accelerate resuspension. If the gel loading buffer is to be diluted, add the H2O to the pellets first. Vortex the pellets to remove them from the sides of the tubes, and solubilize them in the buffer. The pellets should then be heated to 85–95°C for 2–10 minutes to denature the nucleic acid strands. The vortexing and heating steps can be repeated until the pellets appear to have dissolved. Trituration (by pipetting up and down) with a pipettor may also aid in solubilization and permit detection of any undissolved particles. When finished heating, the tubes are placed on ice. They can be microfuged again briefly (3 seconds) to collect any condensation. One can insure the complete transfer of radioactive samples to the gel by using a hand-held Geiger counter to monitor the empty tube after loading the sample on the gel.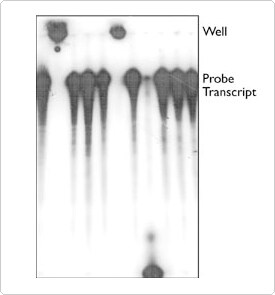
Flushing the wells of the gel
Special situations
Sample tubes
Infrequently, despite care in pellet resuspension and flushing of gel wells, labeled material will still become stuck in the wells. We have found that an occasional Eppendorf tube will contain residue that can cause this to happen. Figure 1 shows a radiolabeled RNA probe that was aliquotted to 11 fresh Eppendorf tubes containing gel loading buffer, heated, and subsequently loaded onto a thoroughly flushed denaturing polyacrylamide gel. Two of the samples remained in the wells. Note that another sample was totally degraded, suggesting that an occasional tube may contain some ribonuclease. If a percentage of your samples continually remains in the wells of the gel, or shows degradation irrespective of the technique being used, we suggest that you try changing the supplier of your sample tubes. Autoclaving silanized Eppendorf tubes also appears to leave residue that can cause well hang up.
Gel purification
Gel purification of probes used in nuclease protection studies can help eliminate radioactivity left in the wells. Much of this signal is presumed to be due to residual unincorporated radiolabeled nucleotides that stick to the wells, as the intensity of this signal is usually independent of that produced by the target hybridized to probe. Figure 2 compares a mouse ß-actin RNA probe precipitated twice with NH4OAc and EtOH after synthesis to a probe purified on a gel. Both probes were then used in a ribonuclease protection assay to protect ß-actin transcripts in 10 µg of total mouse liver RNA. When the digestion products were loaded on a gel, the sample wells in which non-gel purified probe products were loaded showed residual radioactivity. Note, however, that there is no loss of protected fragment signal in these lanes compared to those in which gel purified probes were used.
Carriers and protein molecules
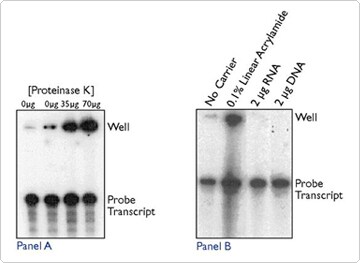
Protein and carrier molecules can lead to hang up in the wells. Crude RNA preparations containing significant amounts of protein or chromosomal DNA can form insoluble complexes upon precipitation that remain in the wells. Proteinase K treatment followed by phenol extraction or DNase treatment will often dissolve these complexes. However, addition of enzyme to the sample to degrade contaminating protein or DNA can also produce this effect. Figure 3A shows a gel where proteinase K has been added to replicate aliquots of an RNA probe transcript. As the concentration of proteinase K is increased (from 0 µg proteinase K in lanes 1 and 2, 35 µg in lane 3, to 70 µg in lane 4), the amount of radiolabel remaining in the wells also increases. Note again that the probe transcript signal remains unchanged.
Linear acrylamide, glycogen, RNA, and DNA are all used to enhance quantitative precipitation of small amounts of nucleic acids from dilute solutions. Since linear acrylamide is the only one of these molecules purified from a non-biological source, it is considered the carrier of choice when used in downstream applications in which contaminating nucleic acids could yield spurious products (PCR and RT-PCR applications) or compete for labeling (end-labeling with terminal transferase). However, linear acrylamide can cause well hang up, whereas RNA and DNA carriers do not (Figure 3B).
APS capsules
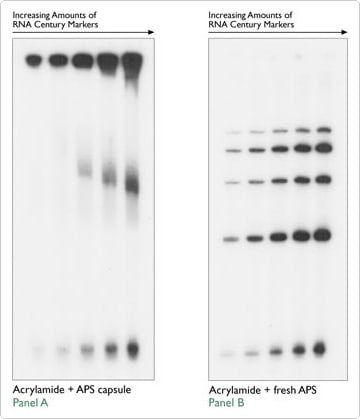
10% ammonium persulfate (APS) aliquots can be purchased in gelatin capsules as a polymerization catalyst for polyacrylamide gels. However, in Figure 4, Dr. Scott Nelson (University of Iowa, personal communication) demonstrates that these capsules seriously impeded sample migration. When increasing amounts of Ambion's RNA Century Markers were run on a denaturing acrylamide gel made with the 10% APS capsules (Figure 4A), their migration pattern was strongly affected. However, when the same dilution series was run on a gel made with fresh 10% APS, the size markers migrated as expected (Figure 4B).
Summary
Sample hang up is usually sporadic and can be caused by a variety of factors. This makes the problem particularly hard to troubleshoot. In this article, we point out some of the more common causes of sample hang up, and make suggestions to help you avoid or overcome this problem. However, we realize that these procedures will not eliminate all sample hang up in the wells. If you can attribute well hang up to a specific factor or situation, we would appreciate hearing about it so that we can pass on your data and experiences to other customers. Please contact our Technical Support Department if you have data you wish to share, or have a hang-up/problem that this article does not adequately address.