Search
pSilencer™ 5.1 Retro System
The pSilencer™ 5.1 Retro System enables researchers to study the long-term effects of reducing the expression of specific genes in cell culture models. These retroviral vectors make use of H1 or U6 Polymerase III promoters to stably express siRNAs, even in difficult-to-transfect cell types. This technology expands RNA interference research to address long-term alteration of the expression of genes that cause disease phenotypes.
Introduction: Getting siRNAs into Cells with Viruses
The DNA-mediated siRNA expression techniques can be divided into viral and nonviral methods. Both involve the expression of a short hairpin RNA transcript that is processed endogenously to an siRNA. Although the nonviral mediated approach is effective for transient RNA interference experiments, low transfection efficiency is a commonly encountered obstacle for production of stable siRNA expressing cell lines, and it presents severe limitations for performing animal studies. (In general, chemically synthesized siRNAs are a better choice for transient experiments in most immortalized cell lines.) In contrast, viral approaches facilitate effective siRNA delivery into many difficult-to-transfect cell types, including primary cells, because infection is a much more efficient delivery mechanism than transfection. In addition, since retroviruses typically integrate into the host’s genome, long-term, stable reduction of gene expression is relatively easy to achieve with these constructs.
Several groups, including Ambion, offer adenoviral, retroviral, and other viral vectors for siRNA expression. For example, Ambion's pSilencer adeno 1.0-CMV System, Cat# 5790, offers an adenoviral-mediated approach to deliver siRNA into cells and animals. p Silencer adeno vectors are ideal for transient siRNA expression in hard-to-transfect cells and in animal tissue.
Now Ambion introduces a retroviral system ( pSilencer 5.1 Retro System) that facilitates stable siRNA expression for studies in mammalian systems (see sidebar, Production of Retroviruses as a Research Tool). The p Silencer retroviral vectors effectively induce gene silencing through efficient siRNA delivery into a wide variety of cells, including hard-to-transfect primary cells. This system is also ideal for long-term, stable knockdown of gene expression, which is critical for understanding and studying how reducing expression of disease-relevant genes cause disease phenotypes over extended periods of time.
This article describes how Ambion’s retroviral system was used to elicit RNAi in both transformed and primary cell types. Strong gene silencing was observed even in an extremely difficult-to-transfect primary cell type, NHDF-neo cells. The p Silencer 5.1 Retro System was also used for long-term stable reduction of gene expression in an experiment continued for over eight months. Surprisingly, some changes in cellular morphology and differences in growth characteristics became evident only after very prolonged gene silencing.
Choice of Promoter Allows Optimal Gene Knockdown
Ambion provides two pSilencer 5.1 Retro vectors—one that features the polymerase III U6 promoter, and one that includes the polymerase III H1 promoter. siRNAs are expressed differentially from these promoters; thus the choice of promoter can affect the level of gene silencing. To demonstrate this, inserts encoding siRNA hairpins targeting several genes including cyclophilin A, GAPDH, ATM, BRCA-1, and CDK2 were cloned into both U6 and H1 promoter-containing pSilencer retroviral vectors. HeLa cells infected with the resulting viruses were placed under selection with puromycin. Surviving cells were analyzed for reduction in mRNA levels. Figure 1A demonstrates that mRNA expression reduction differences were dependent on which promoter was driving siRNA expression. For example, mRNA levels for ATM and cyclophilin were more strongly reduced when their respective siRNA inserts were expressed from the H1 promoter compared to the U6 promoter. Conversely, BRCA-1 mRNA levels were more strongly reduced when the U6 promoter was used to express BRCA-1 siRNA compared to the H1 promoter.
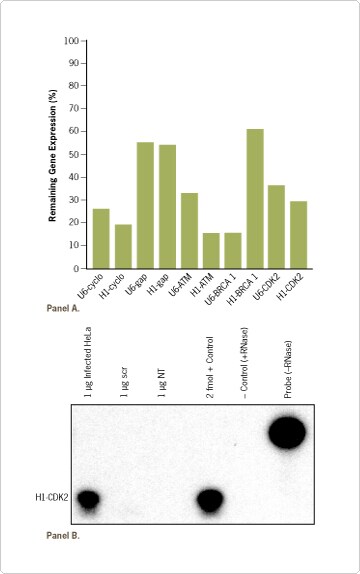
Figure 1. Gene Silencing After Retroviral Delivery of siRNA. Retroviral vectors expressing the indicated siRNA inserts from either the H1 or U6 promoter were transfected into a retrovirus packaging cell line in 6 well plates. Three days following infection, virus was collected and used to infect HeLa cells in triplicate. (A) Infected HeLa cells were placed under puromycin selection (4 days). After two months of continued growth, RNA was purified from the cells and analyzed by real-time PCR for target gene expression levels. Data were expressed relative to cells expressing negative control siRNA. Standard deviations were all below 5%. (B) The expression level of CDK2 siRNA regulated by the H1 promoter (H1CDK2) was monitored using the mirVana™ miRNA Isolation and Detection Kits (Ambion).
In order to measure siRNA expression levels in infected cells, small RNAs were purified using the mirVana™ miRNA Isolation Kit (Ambion), and the specific siRNA was identified with the mirVana miRNA Detection Kit (Ambion), a solution-based hybridization assay. As observed in Figure 1B, CDK2 siRNA was robustly expressed. (Note: The correlation between siRNA expression level and subsequent target gene knockdown will differ across siRNAs studied. This is because some siRNAs are more efficient at eliciting effective silencing than others.)
To achieve the greatest amount of siRNA expression and consequent target gene silencing, it may be advantageous to analyze both promoter systems for each gene being studied. Ambion’s new pSilencer 5.1 Retro System provides vectors with each of the polymerase U6 and H1 promoters for this purpose. Because both vectors use the same restriction sites, siRNA-encoding inserts can be readily cloned from one to the other.
Knockdown of Gene Expression in Primary Cell Lines
Once control cells were killed by puromycin, the experimental cells were removed from antibiotic selection (~4 days). A fraction of the cells was harvested to analyze cyclophilin expression in cells that received the cyclophilin siRNA relative to cells that received the negative control siRNA. The remaining cells were propagated in long-term culture. pSilencer retroviral vectors expressing cyclophilin siRNA effectively reduced cyclophilin mRNA levels in both cell types, indicative of effective siRNA delivery and expression (Figure 2). In fact, gene silencing of cyclophilin was more effective in the primary NHDF-neo cells than in the HeLa cells, suggesting that certain inserts may be more effective in one cell line compared to another.
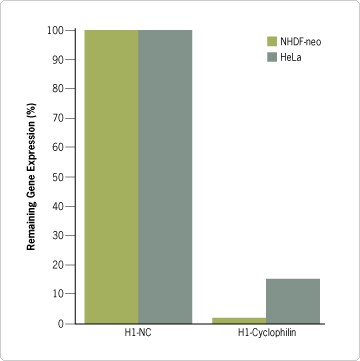
In this experiment, NHDF-neo cells with reduced cyclophilin mRNA levels became “sick” (showed increased cell death), and after two months it was no longer possible to propagate the cells; remaining cells were cultured until they eventually died. In contrast, HeLa cells with reduced cyclophilin expression did not show the same level of cell death and could still be expanded, but the cells exhibited morphological changes (Figure 3C). The morphological differences between the primary NHDF-neo cell cultures and transformed HeLa cell line could be due to differences in cyclophilin reduction, since cyclophilin levels were reduced more effectively in NHDF-neo cells compared to HeLa cells. They could also be due to a biological difference between the two cell types; e.g. their ability to overcome or adapt to reduced cyclophilin expression. Additional experiments using cloned populations of infected cells expressing different levels of siRNA will be required to distinguish between these two scenarios.
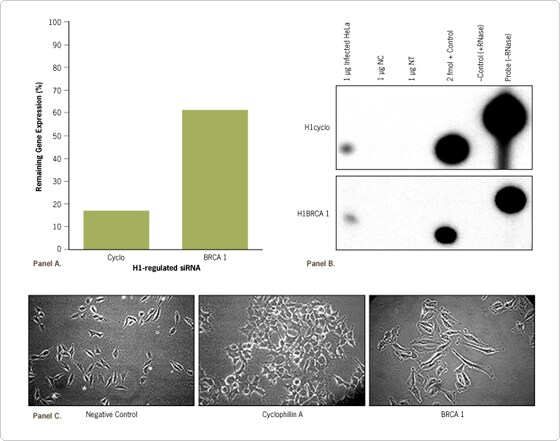
Figure 3. Long-term Reduction in Target Gene Expression Results in Morphological Changes. Retroviral DNA, expressing cyclophilin A, BRCA 1, or a negative control siRNA from the H1 promoter, was transfected into a packaging cell line in a 6 well dish in triplicate. Three days later virus was collected and used to infect HeLa cells. The HeLa cells were placed under puromycin selection (4 days). (A) Cells were periodically analyzed over an 8 month period for gene reduction. Data are depicted as the percent of gene expression compared to cells expressing the negative control siRNA (nc), and standard deviations were below 5%. (B) Cells were also analyzed for expression of siRNA inserts (8 month time point) using the mirVana™ miRNA Detection Kit (Ambion). (C) Morphological changes were observed for cells expressing cyclophilin and BRCA1 siRNAs after 8 months of culture post selection.
Stable Knockdown of Gene Expression
Several interesting observations about cell morphology were noted. Morphological changes were not initially evident even in cells where target gene expression had been reduced for 1–2 months; however, cells in which expression of cyclophilin and BRCA1 had been reduced long-term began to show significant phenotypic differences compared to a stable cell line expressing the negative control siRNA (Figure 3C). Although BRCA1 mRNA levels were 60% of that observed in the cells expressing a negative control siRNA (Figure 3A, B), pronounced morphological phenotypes arose in the BRCA1 siRNA-expressing cells. Thus, relative reduction of mRNA levels by 70% or more is not always necessary to achieve a morphological response. Indeed, long-term reduction of gene expression to more modest levels may mimic the effects seen with heterogeneous disease causing alleles such as BRCA1, where only one of the alleles needs to be mutated to see a disease phenotype, and which often takes extended time to develop.
pSilencer Retro System
Scientific Contributors
Lance P Ford*, Kevin Kelnar, and Lesslie Beauchamp • Ambion, Inc.