Search
Page Contents
Intracellular Ca2+ levels modulate a multitude of vital cellular processes—including gene expression, cell viability, cell proliferation, cell motility, cell shape and volume regulation—thereby playing a key role in regulating cell responses to external signals. These dynamic changes in Ca2+ levels are regulated by ligand-gated and G-protein–coupled ion channels in the plasma membrane and by mobilization of Ca2+ from intracellular stores. The generation of cytosolic Ca2+ spikes and oscillations typically involves the coordinated release and uptake of Ca2+ from these stores, mediated by intracellular Ca2+ channels and their response to several second messengers such as Ca2+ itself, cyclic ADP ribose and inositol triphosphate.![]()
This section includes several Molecular Probes reagents for studying Ca2+ regulation in live cells. Fluorescent nucleotides, including analogs of ATP, ADP, AMP, GTP and GDP, are described in Probes for Protein Kinases, Protein Phosphatases and Nucleotide-Binding Proteins—Section 17.3. Our GTP analogs may be particularly useful in the assay of G-protein–coupled receptors. Probes for Lipid Metabolism and Signaling—Section 17.4 discusses several selective phospholipase substrates, as well as labeled ceramide and sphingomyelin probes.
D-myo-1,4,5-Inositol Triphosphate and Caged D-myo-1,4,5-Inositol Triphosphate
We offer the potassium salt of D-myo-inositol 1,4,5-triphosphate (Ins 1,4,5-P3, I3716) for researchers investigating inositol triphosphate–dependent Ca2+ mobilization and signal transduction mechanisms.![]() Cytoplasmic Ins 1,4,5-P3 is a potent intracellular second messenger that induces Ca2+ release from membrane-bound stores in many tissues. Our Ins 1,4,5-P3 is at least 99% pure, as determined by paper chromatography and by 1H NMR and 31P NMR.
Cytoplasmic Ins 1,4,5-P3 is a potent intracellular second messenger that induces Ca2+ release from membrane-bound stores in many tissues. Our Ins 1,4,5-P3 is at least 99% pure, as determined by paper chromatography and by 1H NMR and 31P NMR.
NPE-caged Ins 1,4,5-P3 can be used to generate rapid and precisely controlled release of Ins 1,4,5-P3 in intact cells and is widely employed in studies of Ins 1,4,5-P3–mediated second messenger pathways.![]() Our NPE-caged Ins 1,4,5-P3 (I23580) is a mixture of the physiologically inert, singly esterified P4 and P5 esters and does not contain the somewhat physiologically active P1 ester. NPE-caged Ins 1,4,5-P3 exhibits essentially no biological activity prior to photolytic release of the biologically active Ins 1,4,5-P3.
Our NPE-caged Ins 1,4,5-P3 (I23580) is a mixture of the physiologically inert, singly esterified P4 and P5 esters and does not contain the somewhat physiologically active P1 ester. NPE-caged Ins 1,4,5-P3 exhibits essentially no biological activity prior to photolytic release of the biologically active Ins 1,4,5-P3.
Fluorescent Heparin
Fluorescein-labeled heparin (H7482) is a useful tool for studying binding of this mucopolysaccharide in cells and tissues.![]() In addition to its well-known anticoagulant activity,
In addition to its well-known anticoagulant activity,![]() heparin binds to the Ins 1,4,5-P3 receptor and inhibits the biological cascade of events mediated by Ins 1,4,5-P3.
heparin binds to the Ins 1,4,5-P3 receptor and inhibits the biological cascade of events mediated by Ins 1,4,5-P3.![]() Heparin also binds to thrombin
Heparin also binds to thrombin ![]() and Alzheimer's tau protein,
and Alzheimer's tau protein,![]() as well as to blood vessel–associated proteins such as laminin and fibronectin.
as well as to blood vessel–associated proteins such as laminin and fibronectin.![]() Fluorescence polarization assays using fluorescein-labeled heparin as a tracer provide quantitative assessments of these binding interactions.
Fluorescence polarization assays using fluorescein-labeled heparin as a tracer provide quantitative assessments of these binding interactions.![]() Fluorescein-labeled heparin has also been used to assess the efficacy of transdermal delivery of heparin by pulsed current iontophoresis as a potential alternative to conventional subcutaneous injections.
Fluorescein-labeled heparin has also been used to assess the efficacy of transdermal delivery of heparin by pulsed current iontophoresis as a potential alternative to conventional subcutaneous injections.![]()
Caged ions and caged chelators can be used to influence the ionic composition of both solutions and cells, particularly for ions such as Ca2+ that are present at low concentrations. The properties and uses of caged probes are described in Photoactivatable Reagents, Including Photoreactive Crosslinkers and Caged Probes—Section 5.3.
NP-EGTA: A Caged Ca2+ Reagent
Developed by Ellis-Davies and Kaplan, the photolabile chelator o-nitrophenyl EGTA (NP-EGTA) exhibits a high selectivity for Ca2+, a dramatic 12,500-fold decrease in affinity for Ca2+ upon UV illumination (its Kd increases from 80 nM to >1 mM) and a high photochemical quantum yield ![]() (~0.2). Furthermore, with a Kd for Mg2+ of 9 mM, NP-caged EGTA does not perturb physiological levels of Mg2+. We offer both the potassium salt (N6802) and the acetoxymethyl (AM) ester (N6803) of NP-EGTA. The NP-EGTA salt can be complexed with Ca2+ to generate a caged calcium complex that will rapidly deliver Ca2+ upon photolysis (Figure 17.2.1). The cell-permeant AM ester of NP-EGTA does not bind Ca2+ unless the AM esters are removed. It can potentially serve as a photolabile buffer in cells because, once converted to NP-EGTA by intracellular esterases, it will bind Ca2+ with high affinity until photolyzed with UV light. NP-EGTA has been used to measure the calcium buffering capacity of cells.
(~0.2). Furthermore, with a Kd for Mg2+ of 9 mM, NP-caged EGTA does not perturb physiological levels of Mg2+. We offer both the potassium salt (N6802) and the acetoxymethyl (AM) ester (N6803) of NP-EGTA. The NP-EGTA salt can be complexed with Ca2+ to generate a caged calcium complex that will rapidly deliver Ca2+ upon photolysis (Figure 17.2.1). The cell-permeant AM ester of NP-EGTA does not bind Ca2+ unless the AM esters are removed. It can potentially serve as a photolabile buffer in cells because, once converted to NP-EGTA by intracellular esterases, it will bind Ca2+ with high affinity until photolyzed with UV light. NP-EGTA has been used to measure the calcium buffering capacity of cells.![]()

Figure 17.2.1 NP-EGTA (N6802) complexed with Ca2+. Upon illumination, this complex is cleaved to yield free Ca2+ and two iminodiacetic acid photoproducts. The affinity of the photoproducts for Ca2+ is ~12,500-fold lower than that of NP-EGTA.
DMNP-EDTA: A Caged Ca2+ Reagent
The first caged Ca2+ reagent described by Ellis-Davies and Kaplan was 1-(4,5-dimethoxy-2-nitrophenyl) EDTA (DMNP-EDTA, D6814), which they named DM-Nitrophen ![]() (now a trademark of Calbiochem-Novabiochem Corp.). Because its structure better resembles that of EDTA than EGTA, we named it as a caged EDTA derivative (Figure 17.2.2). Upon illumination, DMNP-EDTA's Kd for Ca2+ increases from 5 nM to 3 mM. Thus, photolysis of DMNP-EDTA complexed with Ca2+ results in a pulse of free Ca2+. Furthermore, DMNP-EDTA has significantly higher affinity for Mg2+ (Kd = 2.5 µM) than does NP-EGTA
(now a trademark of Calbiochem-Novabiochem Corp.). Because its structure better resembles that of EDTA than EGTA, we named it as a caged EDTA derivative (Figure 17.2.2). Upon illumination, DMNP-EDTA's Kd for Ca2+ increases from 5 nM to 3 mM. Thus, photolysis of DMNP-EDTA complexed with Ca2+ results in a pulse of free Ca2+. Furthermore, DMNP-EDTA has significantly higher affinity for Mg2+ (Kd = 2.5 µM) than does NP-EGTA ![]() (Kd = 9 mM). The photolysis product's Kd for Mg2+ is ~3 mM, making DMNP-EDTA an effective caged Mg2+ source, in addition to its applications for photolytic Ca2+ release.
(Kd = 9 mM). The photolysis product's Kd for Mg2+ is ~3 mM, making DMNP-EDTA an effective caged Mg2+ source, in addition to its applications for photolytic Ca2+ release.![]() Photorelease of Ca2+ has been shown to occur in <180 microseconds, with even faster photorelease of Mg2+.
Photorelease of Ca2+ has been shown to occur in <180 microseconds, with even faster photorelease of Mg2+.![]() Two reviews by Ellis-Davies discuss the uses and limitations of DMNP-EDTA.
Two reviews by Ellis-Davies discuss the uses and limitations of DMNP-EDTA.![]()
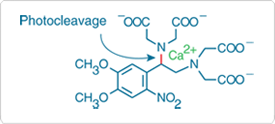
Figure 17.2.2 DMNP-EDTA (D6814) complexed with Ca2+. Upon illumination, this complex is cleaved to yield free Ca2+ and two iminodiacetic acid photoproducts. The affinity of the photoproducts for Ca2+ is ~600,000-fold lower than that of DMNP-EDTA.
Diazo-2: A Photoactivatable Ca2+ Knockdown Reagent
In contrast to NP-EGTA and DMNP-EDTA, diazo-2 (D3034) is a photoactivatable Ca2+ scavenger. Diazo-2, which was introduced by Adams, Kao and Tsien,![]() is a relatively weak chelator (Kd for Ca2+ = 2.2 µM). Following flash photolysis at ~360 nm, however, cytosolic free Ca2+ rapidly binds to the diazo-2 photolysis product, which has a high affinity for Ca2+ (Kd = 73 nM). Microinjecting a relatively low concentration of fluo-3, fluo-4, or one of the Calcium Green or Oregon Green 488 BAPTA indicators (Fluorescent Ca2+ Indicators Excited with Visible Light—Section 19.3), along with a known quantity of diazo-2, permits measurement of the extent of depletion of cytosolic Ca2+ following photolysis.
is a relatively weak chelator (Kd for Ca2+ = 2.2 µM). Following flash photolysis at ~360 nm, however, cytosolic free Ca2+ rapidly binds to the diazo-2 photolysis product, which has a high affinity for Ca2+ (Kd = 73 nM). Microinjecting a relatively low concentration of fluo-3, fluo-4, or one of the Calcium Green or Oregon Green 488 BAPTA indicators (Fluorescent Ca2+ Indicators Excited with Visible Light—Section 19.3), along with a known quantity of diazo-2, permits measurement of the extent of depletion of cytosolic Ca2+ following photolysis.![]() Intracellular loading of NP-EGTA, DMNP-EDTA and diazo-2 is best accomplished by patch pipette infusion with the carboxylate salt form of the caged compound added to the internal pipette solution at 1–10 mM. These reagents are increasingly being applied in vivo for controlled intervention in calcium-regulated fundamental processes in neurobiology
Intracellular loading of NP-EGTA, DMNP-EDTA and diazo-2 is best accomplished by patch pipette infusion with the carboxylate salt form of the caged compound added to the internal pipette solution at 1–10 mM. These reagents are increasingly being applied in vivo for controlled intervention in calcium-regulated fundamental processes in neurobiology ![]() and developmental biology.
and developmental biology.![]()
Thapsigargin and Fluorescent Thapsigargin
Thapsigargin is a naturally occurring sesquiterpene lactone isolated from the umbelliferous plant Thapsia garganica.![]() This tumor promoter releases Ca2+ from intracellular stores by specifically inhibiting the sarcoplasmic reticulum Ca2+-ATPase
This tumor promoter releases Ca2+ from intracellular stores by specifically inhibiting the sarcoplasmic reticulum Ca2+-ATPase ![]() (SERCA); it does not directly affect plasma membrane Ca2+-ATPases, Ins 1,4,5-P3 production or protein kinase C activity.
(SERCA); it does not directly affect plasma membrane Ca2+-ATPases, Ins 1,4,5-P3 production or protein kinase C activity.![]()
Thapsigargin is available in 1 mg units (T7458) and specially packaged in 20 vials containing 50 µg each (T7459). We have also prepared the green-fluorescent BODIPY FL thapsigargin (B7487) and red-fluorescent BODIPY TR-X thapsigargin (B13800). BODIPY FL thapsigargin has proven useful for imaging the intracellular localization of thapsigargin during store-operated calcium entry (SOCE) ![]() and for imaging SERCA depletion in injured sensory neurons.
and for imaging SERCA depletion in injured sensory neurons.![]()
Luminescent Calcium Analog
The trivalent lanthanide terbium (III), which is supplied as its chloride salt (T1247), is a luminescent analog of Ca2+ that can be used to study structure–function relationships in Ca2+-binding proteins such as calmodulin, oncomodulin, lactalbumin and ATPases.![]() The long-lived luminescence of Tb3+ has also been use to probe Ca2+-binding sites of alkaline phosphatase,
The long-lived luminescence of Tb3+ has also been use to probe Ca2+-binding sites of alkaline phosphatase,![]() glutamine synthetase,
glutamine synthetase,![]() integrins,
integrins,![]() protein kinase C
protein kinase C ![]() and ryanodine-sensitive Ca2+ channels.
and ryanodine-sensitive Ca2+ channels.![]() Tb3+ reportedly binds most strongly to the I and II sites of calmodulin.
Tb3+ reportedly binds most strongly to the I and II sites of calmodulin.![]()
| Cat # | MW | Storage | Soluble | Abs | EC | Em | Solvent | Notes |
|---|---|---|---|---|---|---|---|---|
| B7487 | 854.75 | FF,D,L | DMSO | 503 | 85,000 | 511 | MeOH | |
| B13800 | 1100.04 | FF,D,L | DMSO | 589 | 62,000 | 616 | MeOH | |
| D3034 | 710.86 | F,D,LL | pH >6 | 369 | 18,000 | none | pH 7.2 | 1, 3, 4 |
| D6814 | 473.39 | D,LL | DMSO | 348 | 4200 | none | pH 7.2 | 1, 4, 5 |
| H7482 | ~18,000 | FF,D,L | H2O | 493 | ND | 514 | pH 8 | 6, 7 |
| I3716 | 648.64 | F,D | H2O | <250 | none | |||
| I23580 | 872.82 | FF,D,LL | H2O | 264 | 4200 | none | H2O | 1, 2, 8 |
| N6802 | 653.81 | FF,D,LL | pH >6 | 260 | 3500 | none | pH 7.2 | 1, 2, 4, 9 |
| N6803 | 789.70 | FF,D,LL | DMSO | 250 | 4200 | none | MeCN | 10, 11 |
| T1247 | 373.38 | D | H2O | 270 | 4700 | 545 | H2O | 12, 13 |
| T7458 | 650.76 | F,D | DMSO, EtOH | <300 | none | |||
| T7459 | 650.76 | F,D | DMSO, EtOH | <300 | none | |||
| ||||||||
For Research Use Only. Not for use in diagnostic procedures.