Search
Carbodiimide Crosslinker Chemistry
View and select products
- EDC Carbodiimide Crosslinker
- NHS and Sulfo-NHS (N-hydroxysuccinimide)
- KLH and EDC Conjugation Kit
- BSA and EDC Conjugation Kit
- Blue Carrier and EDC Conjugation Kit
- Amine-biotin Compounds (for carbonyl biotinylation)
- Amine-PEG Compounds (for carbonyl pegylation)
Bioconjugation and crosslinking technical handbook
Learn how to optimize your bioconjugation strategies with our updated Bioconjugation and crosslinking technical handbook. This easy-to-use guide overviews our portfolio of reagents for bioconjugation, crosslinking, biotinylation, and modification of proteins and peptides.
Achieve the most efficient modification for your typical applications including:
- Protein and peptide biotinylation
- Antibody labeling with fluorophores and biotin
- Immobilizing biomolecules to surfaces
- Capturing protein interactions
Introduction
Carboxyl-reactive crosslinker reactive groups
Very few chemical groups are known to provide specific and practical conjugation to carboxylic acids (–COOH), such as occur in proteins and many other biomolecules. Certain diazomethane and diazoacetyl reagents have been used to derivatize small compounds for analysis by HPLC or for fluorescent labeling. Carbonyldiimidazole (CDI) can be used in non-aqueous conditions to activate carboxylic acids for direct conjugation to primary amines (–NH2) via amide bonds.
Carbodiimide compounds provide the most popular and versatile method for labeling or crosslinking to carboxylic acids. The most readily available and commonly used carbodiimides are the water-soluble EDC for aqueous crosslinking and the water-insoluble DCC for non-aqueous organic synthesis methods.


Chemical structures of carbodiimides EDC and DCC. EDC (also called EDAC) is 1-ethyl-3-(-3-dimethylaminopropyl) carbodiimide hydrochloride, MW 191.70. DCC is N', N’-dicyclohexyl carbodiimide, MW 206.32.
Carbodiimide conjugation, as with CDI-mediated conjugation, works by activating carboxyl groups for direct reaction with primary amines via amide bond formation. Because no portion of their chemical structure becomes part of the final bond between conjugated molecules, carbodiimides are considered zero-length carboxyl-to-amine crosslinkers.
Bioconjugate Techniques, 3rd Edition
Bioconjugate Techniques, 3rd Edition (2013) by Greg T. Hermanson is a major update to a book that is widely recognized as the definitive reference guide in the field of bioconjugation.
Bioconjugate Techniques is a complete textbook and protocols-manual for life scientists wishing to learn and master biomolecular crosslinking, labeling, and immobilization techniques that form the basis of many laboratory applications. The book is also an exhaustive and robust reference for researchers looking to develop novel conjugation strategies for entirely new applications. It also contains an extensive introduction to the field of bioconjugation that covers all of the major applications of the technology used in diverse scientific disciplines as well as containing tips for designing the optimal bioconjugate for any purpose.

EDC reaction chemistry
EDC reacts with carboxylic acid groups to form an active O-acylisourea intermediate that is easily displaced by nucleophilic attack from primary amino groups in the reaction mixture. The primary amine forms an amide bond with the original carboxyl group, and an EDC by-product is released as a soluble urea derivative. The O-acylisourea intermediate is unstable in aqueous solutions; failure to react with an amine results in hydrolysis of the intermediate, regeneration of the carboxyls, and the release of an N-unsubstituted urea.
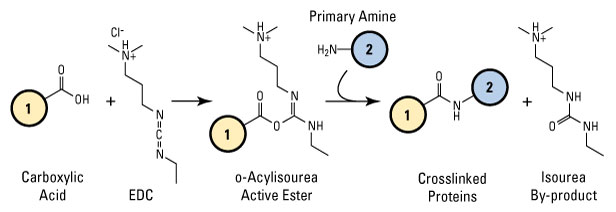
EDC (carbodiimide) crosslinking reaction scheme. Carboxyl-to-amine crosslinking with the popular carbodiimide, EDC. Molecules (1) and (2) can be peptides, proteins or any chemicals that have respective carboxylate and primary amine groups. When they are peptides or proteins, these molecules are tens-to-thousands of times larger than the crosslinker and conjugation arms diagrammed in the reaction.
EDC crosslinking is most efficient in acidic (pH 4.5) conditions and must be performed in buffers devoid of extraneous carboxyls and amines. MES buffer (4-morpholinoethanesulfonic acid) is a suitable carbodiimide reaction buffer. Phosphate buffers and neutral pH (up to 7.2) conditions are compatible with the reaction chemistry, albeit with lower efficiency; increasing the amount of EDC in a reaction solution can compensate for the reduced efficiency.
N-hydroxysuccinimide (NHS) or its water-soluble analog (Sulfo-NHS) is often included in EDC coupling protocols to improve efficiency or create dry-stable (amine-reactive) intermediates. EDC couples NHS to carboxyls, forming an NHS ester that is considerably more stable than the O-acylisourea intermediate while allowing for efficient conjugation to primary amines at physiologic pH.

Sulfo-NHS plus EDC (carbodiimide) crosslinking reaction scheme. Carboxyl-to-amine crosslinking using the carbodiimide EDC and Sulfo-NHS. Addition of NHS or Sulfo-NHS to EDC reactions (bottom-most pathway) increases efficiency and enables molecule (1) to be activated for storage and later use.
EDC is also capable of activating phosphate groups in the presence of imidazole for conjugation to primary amines. The method is sometimes used to modify, label, crosslink or immobilize oligonucleotides through their 5' phosphate groups.
Learn more
- Amine Reactive Crosslinker Chemistry
- Tech Tip #30: Modify and label oligonucleotide 5’phosphate groups
- Crosslinking Applications
The ability to crosslink primary amines to carboxylic acid groups using EDC is a powerful and versatile tool for crosslinking peptides and proteins, preparing biomolecular probes, and immobilizing macromolecules for use in numerous protein and cell biology detection and analysis methods.
Of course, peptides and proteins contain both primary amines and carboxylic acids (N- and C-termini, respectively, as well as in the side-chain of certain amino acids). Thus, EDC enables peptides and proteins to be easily conjugated to one another or to any compounds or solid surfaces that bear either carboxyl or amino groups.
1. Peptide conjugation to carrier proteins
Because peptides and proteins contain both carboxylates and amines, EDC-mediated crosslinking usually causes random polymerization of polypeptides. This outcome is desirable when preparing immunogens for use in antibody production because it allows peptide antigens to be polymerized and conjugated at high densities onto immunogenic carrier proteins, such as KLH or BSA.

Coupling of peptide to a carrier protein using EDC. Here “C” represents the carrier protein and “P” represents the peptide.
Learn more
Select products
2. Label carboxyl groups using amine-containing compounds
EDC provides the only method for labeling or crosslinking to carboxyl groups of peptides or proteins (i.e., the C-terminus and side chains of glutamic acid and aspartic acid). To accomplish this without also reacting to a significant number of primary amines on the peptide or protein, one must supply a large molar excess of the desired amine-containing molecule. For example, to biotinylate only the C-terminus of a peptide, one would combine the peptide with something like a 100-fold molar excess of an amine-containing biotin compound before adding the appropriate amount of EDC. Most of the amines encountered by EDC-activated carboxylic acid groups of the peptide would be those of the biotin compound, so peptide-to-biotin conjugation would predominate over peptide-to-peptide conjugation.

Amine-PEG2-Biotin is an example of an amine containing biotinylation reagent
Learn more
Select products
- EDC
- Amine-Biotin Compounds (for carbonyl biotinylation)
- Amine-PEG Compounds (for carbonyl pegylation)
3. Immobilize peptides for affinity purification
Proteins are typically immobilized or crosslinked via primary amines or sulfhydryl groups rather than through carboxylates. By contrast, peptides (and other small carboxyl-containing molecules) are often immobilized using EDC to polymerize and conjugate them to an amine-derivatized surface material or solid support such as agarose resin. For example, Thermo Scientific CarboxyLink Coupling Resin is 4% beaded agarose that has been modified with diaminodipropylamine (DADPA); it provides the necessary amines for conjugation of peptide carboxylates at the end of a long spacer arm. Peptides are frequently immobilized to agarose beads by this method for antigen-specific affinity purification of antibodies following immunization of animals with peptide-KLH conjugates.

Immobilization of peptides using Carboxylink Coupling Resin
4. Attach peptides to surface materials
Besides agarose beads, many other solid materials are used as platforms to immobilizing molecules for assay methods. Primary amines and carboxylic acids are the most common foundational surface-derivatives for solid particles such as magnetic beads or glass slides. Aminosilane compounds such as 3-aminopropyl-triethoxysilane (APTS or APS) are popular reagents for coating glass (borosilicate) surfaces with primary amines. EDC is then one important means for covalently attaching peptides or other compounds to the surface material.
Learn more
- Tech Tip #1 Attach to Glass Surfaces
- Tech Tip # 2Attach to Gold Surfaces
- Tech Tip #5 Attach Antibodies to Glass, Silica, Quartz
DCC reaction chemistry and applications
DCC (dicyclohexyl carbodiimide) crosslinks carboxylic acids to primary amines in the same manner as EDC (see reaction schemes above). However, because DCC is not aqueous-soluble, it is primarily used in manufacturing and organic synthesis applications rather than in the typical protein research biology lab. For example, most commercially available, ready-to-use NHS-ester crosslinkers and labeling reagents are manufactured using DCC. Because water is excluded, the resulting NHS ester can be prepared and stabilized as a dried powder without appreciable hydrolysis. DCC is also commonly used in commercial peptide synthesis operations.

Structure of DCC
- Samavati, A. et al., (2020) Sustainable and fast saliva-based COVID-19 virus diagnosis kit using a novel GO-decorated Au/FBG sensor. Chemical Engineering Journal Advance Online Publication. https://doi.org/10.1016/j.cej.2020.127655
- Quantitative proteomics targeting classes of motif-containing peptides using immunoaffinity-based mass spectrometry. Authors: Olsson N, James P, Borrebaeck CA, Wingren C. Journal: Mol Cell Proteomics 2012; (11):8 342-354
- Quantitative proteomics targeting classes of motif-containing peptides using immunoaffinity-based mass spectrometry. Authors: Olsson N, James P, Borrebaeck CA, Wingren C. Journal: Mol Cell Proteomics 2012; (11):8 342-354
- Enhanced leukemia cell detection using a novel magnetic needle and nanoparticles. Authors: Jaetao J. E. et al. Journal: Cancer Res (2009) 69:8310-6
- Metabolism of vertebrate amino sugars with N-glycolyl groups: elucidating the intracellular fate of the non-human sialic acid N-glycolylneuraminic acid. Authors: Bergfeld AK, Pearce OM, Diaz SL, Pham T, Varki A. Journal: J Biol Chem 2012; (287):34 28865-28881
For Research Use Only. Not for use in diagnostic procedures.
