Search
Assays of Volume Change, Membrane Fusion and Membrane Permeability—Note 14.3
Assays of membrane fusion report either the mixing or leakage of the aqueous contents of the fused entities (described here) or the mixing of membrane lipids (Lipid-Mixing Assays of Membrane Fusion—Note 13.1). Leakage of aqueous contents from cells or vesicles as a result of lysis, fusion or physiological permeability can be detected fluorometrically using low molecular weight soluble tracers. Assays designed to detect solution mixing often rely on a fluorophore and quencher pair, whereas assays that detect solution leakage into the external medium typically exploit the self-quenching properties of a fluorophore.
Fluorescence Quenching Assays with ANTS/DPX
Originally developed by Smolarsky and co-workers to follow complement-mediated immune lysis,![]() the ANTS/DPX fluorescence quenching assay has since been widely used to detect membrane fusion.
the ANTS/DPX fluorescence quenching assay has since been widely used to detect membrane fusion.![]() This assay is based on the collisional quenching of the polyanionic fluorophore ANTS by the cationic quencher DPX (Figure 1). Separate vesicle populations are loaded with 25 mM ANTS (A350) in 40 mM NaCl and 90 mM DPX (X1525).
This assay is based on the collisional quenching of the polyanionic fluorophore ANTS by the cationic quencher DPX (Figure 1). Separate vesicle populations are loaded with 25 mM ANTS (A350) in 40 mM NaCl and 90 mM DPX (X1525).
Vesicle fusion results in quenching of ANTS fluorescence monitored at 530 nm, with excitation at 360 nm. External leakage of vesicle contents does not cause quenching due to the accompanying high dilution of ANTS and DPX. For assays of vesicle leakage, ANTS (12.5 mM) and DPX (45 mM) can be co-encapsulated into liposomes; upon dilution into surrounding medium, ANTS fluorescence will increase because quenching by DPX will be diminished.
Examples employing ANTS/DPX quenching include studies of the role of amino lipids ![]() and phospholipid asymmetry
and phospholipid asymmetry ![]() in membrane fusion; glycoprotein-mediated viral fusion with membranes;
in membrane fusion; glycoprotein-mediated viral fusion with membranes;![]() and the interactions of vesicle membranes with annexins,
and the interactions of vesicle membranes with annexins,![]() HIV fusion peptide
HIV fusion peptide ![]() and apolipoprotein.
and apolipoprotein.![]()
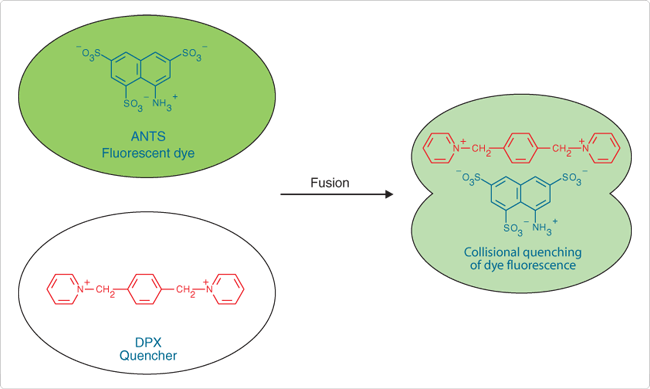
Figure 1. Pictorial representation of the ANTS/DPX vesicle-fusion assay.
Additional Dye/Quencher Pairs
The fluorescence of HPTS and pyrenetetrasulfonic acid is also effectively quenched by DPX.![]() Thallium (Tl+) and cesium (Cs+) ions quench the fluorescence of ANTS, pyranine (HPTS, H348), pyrenetetrasulfonic acid (P349) and some other polyanionic fluorophores. A review by Garcia
Thallium (Tl+) and cesium (Cs+) ions quench the fluorescence of ANTS, pyranine (HPTS, H348), pyrenetetrasulfonic acid (P349) and some other polyanionic fluorophores. A review by Garcia ![]() describes how fluorescence quenching with a variety of dye/quencher pairs can be used to determine transmembrane ion permeability.
describes how fluorescence quenching with a variety of dye/quencher pairs can be used to determine transmembrane ion permeability.
Fluorescence Enhancement Assays with Tb3+/DPA
In the Tb3+/dipicolinic acid (DPA) assay, which was originally described by Wilschut and Papahadjopoulos,![]() separate vesicle populations are loaded with 2.5 mM TbCl3 (T1247) in 50 mM sodium citrate, or 50 mM DPA in 20 mM NaCl.
separate vesicle populations are loaded with 2.5 mM TbCl3 (T1247) in 50 mM sodium citrate, or 50 mM DPA in 20 mM NaCl.
Vesicle fusion results in formation of Tb3+/DPA chelates that are ~10,000 times more fluorescent than free Tb3+ (Figure 2). Fluorescence of the chelates is detected at 490 nm or 545 nm, with excitation at 276 nm. Including Ca2+ and EDTA in the external medium inhibits formation of the complex outside the fused vesicles. The Tb3+/DPA fluorescence enhancement assay has been used to investigate the role of phospholipid conformation in vesicle fusion ![]() and the interaction of cardiotoxin with phospholipid vesicles.
and the interaction of cardiotoxin with phospholipid vesicles.![]()
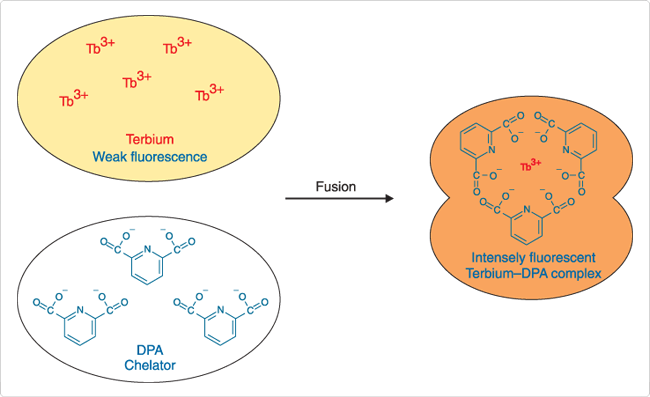
Figure 2. Pictorial representation of the terbium/dipicolinic acid (DPA) fluorescence enhancement assay for vesicle fusion.
Self-Quenching Assays with Fluorescein Derivatives
Fluorescence of carboxyfluorescein (C194, C1904) or calcein (C481) is >95% self-quenched at concentrations >100 mM. Concentrated solutions of these water-soluble dyes are encapsulated in liposomes, which are then separated from any remaining free dye by gel filtration. Upon addition of a fusogen or other permeabilizing agent, dye release is accompanied by an increase in fluorescence (excitation/emission maxima ~490 nm/520 nm). Complete lysis of the liposomes with 0.1% Triton X-100 can be used to determine the assay endpoint. Calcein may be preferred over carboxyfluorescein because of higher net charge and lower pH sensitivity. Note that this assay will detect any process that causes leakage of aqueous contents, including fusion, lysis or permeabilization. In a modification of this assay designed to specifically detect vesicle fusion, a nonfluorescent Co2+ complex of calcein contained in a vesicle is disrupted upon fusion with a second vesicle that delivers EDTA, a chelator of Co2+.![]() Studies employing carboxyfluorescein self-quenching include investigations of interactions of membranes with mycobacterial glycopeptidolipids
Studies employing carboxyfluorescein self-quenching include investigations of interactions of membranes with mycobacterial glycopeptidolipids ![]() and with HIV glycoprotein peptide fragments.
and with HIV glycoprotein peptide fragments.![]()
For Research Use Only. Not for use in diagnostic procedures.