Search
Protein Gel Electrophoresis Chamber Systems

We offer a variety of gel electrophoresis chamber systems that allow you to perform multiple types of gel runs and experiments. You can perform gel transfers in some chambers using optional blot modules. Below is a table to help you choose the right electrophoresis system for your needs.
Which electrophoresis chamber system is right for you?
 |  |  |  |  | |
| Mini Gel Tank | XCell SureLock Mini-Cell | SureLock Tandem Midi Gel Tank | XCell4 SureLock Midi Cell | Tetra Electrophoresis Cores | |
|---|---|---|---|---|---|
| Gel specifications | Gel size: Mini (8 x 8 cm) Gel cassette: 10 x 10 cm Thickness: 1.0 mm or 1.5 mm | Gel size: Mini (8 x 8 cm) Gel cassette: 10 x 10 cm Thickness: 1.0 mm or 1.5 mm | Gel size: Midi (8 x 13 cm) Gel cassette: 10.3 x 15 cm Thickness: 1.0 mm | Gel size: Midi (8 x 13 cm) Gel cassette: 10.3 x 15 cm Thickness: 1.0 mm | Gel size: Mini (8 x 8 cm) Gel cassette: 10 x 10 cm Thickness: 1.0 mm or 1.5 mm |
| Capacity | Up to 2 gels | Up to 2 gels | Up to 2 gels | Up to 4 gels | Up to 4 gels |
| Advantages | Unique convenient side-by-side gel loading and separate chambers for each gel | Simple, sturdy apparatus | Easy-to-use apparatus, with separate chambers for each gel, enabling scalable buffer usage. | Reliable separation of up to 4 midi gels in a single run. | Compatible with Bio-Rad Mini-PROTEAN Tetra Cell and with Invitrogen Mini Protein Gels |
| Buffer requirements | 400 mL for each gel | Upper chamber: 200 mL Lower chamber: 600 mL | Upper chamber: ~170 mL (per gel) Lower chamber: ~350 mL (per gel) | Upper chamber: 175 mL (per gel) Lower chamber: 700 mL (with 4 gels) | 750 mL for 2 gels, 1100 mL for 4 gels |
| Unit dimensions | 32 x 11.5 x 16 cm | 14 x 13 x 16 cm | 25 × 17.9 × 17.3 cm | 21 x 19 x 16 cm | 15 x 5.1 x 14.4 cm (primary) 15 x 5.1 x 12.3 cm (companion) |
| Material | Polycarbonate | Polycarbonate | Polycarbonate | Polycarbonate | Polycarbonate |
| Electrode wire | Platinum | Platinum | Platinum | Platinum | Platinum |
| Electrode limits | 500 VDC or 100 Watts | 1,500 VDC or 75 Watts | 600 VDC or 200 Watts | 600 VDC or 200 Watts | 600 VDC or 30 Watts |
| Transfer unit | Mini Blot Module | XCell II Blot Module | SureLock Tandem Midi Blot Module | - | - |
| Transfer capacity | Up to 2 gels | Up to 2 gels | Up to 2 gels | - | - |
| Compatible power supply | PowerEase, Owl systems, for other systems use with Novex Power Supply Adapters (Cat. No. ZA10001) | PowerEase, Owl systems, for other systems use with Novex Power Supply Adapters (Cat. No. ZA10001) | Zoom Dual Power, PowerEase, Owl systems | Zoom Dual Power, PowerEase, Owl systems, for other systems use with Novex Power Supply Adapters (Cat. No. ZA10001) | PowerEase Touch systems, Bio-Rad Power Pac systems |
| Catalog number | A25977 | EI0001 | STM1001 | WR0100 | 1658040 |
Chamber systems for protein electrophoresis
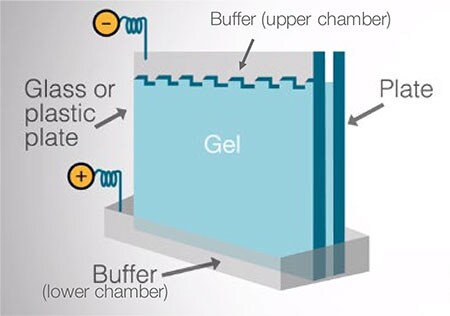
Invitrogen electrophoresis chamber systems are tanks composed of an upper (anode) and lower (cathode) buffer chamber connected by platinum wire electrodes. Gels are held vertically between the buffer chambers during electrophoresis. Applying an electrical field across the buffer chambers forces the migration of proteins into and through the polyacrylamide gel. When current is applied, the smaller molecules migrate more rapidly and the larger molecules migrate more slowly through the gel matrix.
 |
Roles of resistance, voltage, current and power in gel electrophoresis
Resistance
The electrical resistance of the assembled electrophoresis cell is dependent on buffer conductivity, gel thickness, temperature, and the number of gels being run. Although the resistance is determined by the gel system, the resistance varies over the course of the run.
- In discontinuous buffer systems (and to a lesser extent in continuous buffer systems) resistance increases over the course of electrophoresis. This occurs in the Tris-glycine buffer system as highly conductive chloride ions in the gel are replaced by less conductive glycine ions from the running buffer.
- Resistance decreases as the temperature increases.
Voltage
The velocity of an ion in an electric field varies in proportion to the field strength (volts per unit distance). The higher the voltage, the faster an ion moves. For most applications, we recommend a constant voltage setting.
- A constant voltage setting allows the current and power to decrease over the course of electrophoresis, providing a safety margin in case of a break in the system.
- The constant voltage setting does not need adjustment to account for differences in number or thickness of gels being electrophoresed.
Current
For a given gel/buffer system, at a given temperature, current varies in proportion to the field strength (voltage) and cross-sectional area (thickness and number of gels). When using a constant current setting, migration starts slow, and accelerates over time, thus favoring stacking in discontinuous gels. When running under constant current, set a voltage limit on the power supply at, or slightly above the maximum expected voltage to avoid unsafe conditions. At constant current, voltage increases as resistance increases. If a local fault condition occurs (e.g., a bad connection), high local resistance may cause the voltage to reach the maximum for the power supply, leading to overheating and damage of the electrophoresis cell.
Power
Wattage measures the rate of energy conversion, which is manifested as heat generated by the system. Using constant power helps ensure that the total amount of heat generated by the system remains constant throughout the run but results in variable mobility since voltage increases and current decreases over the course of the run. Constant power is typically used when using IEF strips. When using constant power, set the voltage limit slightly above the maximum expected for the run. High local resistance can cause a large amount of heat to be generated over a small distance, damaging the electrophoresis cell and gels.
Which electrophoresis chamber system is right for you?
 |  |  |  |  | |
| Mini Gel Tank | XCell SureLock Mini-Cell | SureLock Tandem Midi Gel Tank | XCell4 SureLock Midi Cell | Tetra Electrophoresis Cores | |
|---|---|---|---|---|---|
| Gel specifications | Gel size: Mini (8 x 8 cm) Gel cassette: 10 x 10 cm Thickness: 1.0 mm or 1.5 mm | Gel size: Mini (8 x 8 cm) Gel cassette: 10 x 10 cm Thickness: 1.0 mm or 1.5 mm | Gel size: Midi (8 x 13 cm) Gel cassette: 10.3 x 15 cm Thickness: 1.0 mm | Gel size: Midi (8 x 13 cm) Gel cassette: 10.3 x 15 cm Thickness: 1.0 mm | Gel size: Mini (8 x 8 cm) Gel cassette: 10 x 10 cm Thickness: 1.0 mm or 1.5 mm |
| Capacity | Up to 2 gels | Up to 2 gels | Up to 2 gels | Up to 4 gels | Up to 4 gels |
| Advantages | Unique convenient side-by-side gel loading and separate chambers for each gel | Simple, sturdy apparatus | Easy-to-use apparatus, with separate chambers for each gel, enabling scalable buffer usage. | Reliable separation of up to 4 midi gels in a single run. | Compatible with Bio-Rad Mini-PROTEAN Tetra Cell and with Invitrogen Mini Protein Gels |
| Buffer requirements | 400 mL for each gel | Upper chamber: 200 mL Lower chamber: 600 mL | Upper chamber: ~170 mL (per gel) Lower chamber: ~350 mL (per gel) | Upper chamber: 175 mL (per gel) Lower chamber: 700 mL (with 4 gels) | 750 mL for 2 gels, 1100 mL for 4 gels |
| Unit dimensions | 32 x 11.5 x 16 cm | 14 x 13 x 16 cm | 25 × 17.9 × 17.3 cm | 21 x 19 x 16 cm | 15 x 5.1 x 14.4 cm (primary) 15 x 5.1 x 12.3 cm (companion) |
| Material | Polycarbonate | Polycarbonate | Polycarbonate | Polycarbonate | Polycarbonate |
| Electrode wire | Platinum | Platinum | Platinum | Platinum | Platinum |
| Electrode limits | 500 VDC or 100 Watts | 1,500 VDC or 75 Watts | 600 VDC or 200 Watts | 600 VDC or 200 Watts | 600 VDC or 30 Watts |
| Transfer unit | Mini Blot Module | XCell II Blot Module | SureLock Tandem Midi Blot Module | - | - |
| Transfer capacity | Up to 2 gels | Up to 2 gels | Up to 2 gels | - | - |
| Compatible power supply | PowerEase, Owl systems, for other systems use with Novex Power Supply Adapters (Cat. No. ZA10001) | PowerEase, Owl systems, for other systems use with Novex Power Supply Adapters (Cat. No. ZA10001) | Zoom Dual Power, PowerEase, Owl systems | Zoom Dual Power, PowerEase, Owl systems, for other systems use with Novex Power Supply Adapters (Cat. No. ZA10001) | PowerEase Touch systems, Bio-Rad Power Pac systems |
| Catalog number | A25977 | EI0001 | STM1001 | WR0100 | 1658040 |
Chamber systems for protein electrophoresis
Invitrogen electrophoresis chamber systems are tanks composed of an upper (anode) and lower (cathode) buffer chamber connected by platinum wire electrodes. Gels are held vertically between the buffer chambers during electrophoresis. Applying an electrical field across the buffer chambers forces the migration of proteins into and through the polyacrylamide gel. When current is applied, the smaller molecules migrate more rapidly and the larger molecules migrate more slowly through the gel matrix.
 |
Roles of resistance, voltage, current and power in gel electrophoresis
Resistance
The electrical resistance of the assembled electrophoresis cell is dependent on buffer conductivity, gel thickness, temperature, and the number of gels being run. Although the resistance is determined by the gel system, the resistance varies over the course of the run.
- In discontinuous buffer systems (and to a lesser extent in continuous buffer systems) resistance increases over the course of electrophoresis. This occurs in the Tris-glycine buffer system as highly conductive chloride ions in the gel are replaced by less conductive glycine ions from the running buffer.
- Resistance decreases as the temperature increases.
Voltage
The velocity of an ion in an electric field varies in proportion to the field strength (volts per unit distance). The higher the voltage, the faster an ion moves. For most applications, we recommend a constant voltage setting.
- A constant voltage setting allows the current and power to decrease over the course of electrophoresis, providing a safety margin in case of a break in the system.
- The constant voltage setting does not need adjustment to account for differences in number or thickness of gels being electrophoresed.
Current
For a given gel/buffer system, at a given temperature, current varies in proportion to the field strength (voltage) and cross-sectional area (thickness and number of gels). When using a constant current setting, migration starts slow, and accelerates over time, thus favoring stacking in discontinuous gels. When running under constant current, set a voltage limit on the power supply at, or slightly above the maximum expected voltage to avoid unsafe conditions. At constant current, voltage increases as resistance increases. If a local fault condition occurs (e.g., a bad connection), high local resistance may cause the voltage to reach the maximum for the power supply, leading to overheating and damage of the electrophoresis cell.
Power
Wattage measures the rate of energy conversion, which is manifested as heat generated by the system. Using constant power helps ensure that the total amount of heat generated by the system remains constant throughout the run but results in variable mobility since voltage increases and current decreases over the course of the run. Constant power is typically used when using IEF strips. When using constant power, set the voltage limit slightly above the maximum expected for the run. High local resistance can cause a large amount of heat to be generated over a small distance, damaging the electrophoresis cell and gels.
For Research Use Only. Not for use in diagnostic procedures.



