Search
SYTO 59 Nuclear Staining Protocol

Nuclear stain for eukaryotic and prokaryotic cells
The cell-permeant SYTO 59 nucleic acid stain exhibits bright red fluorescence upon binding to nucleic acids. In both live and dead eukaryotic cells, SYTO 59 generally shows cytoplasmic or mitochondrial as well as nuclear staining. In addition, SYTO 59 will stain most live and permeabilized bacteria.
This protocol can be used for:
- Nucleic acid (nuclear) staining in fluorescence microscopy
This protocol should not be used for:
- Flow cytometry
You will need the following for this protocol:
- Cells growing in culture
- SYTO 59 Red Fluorescent Nucleic Acid Stain (Cat. No. S11341)
- Fluorescence microscope
Protocol
Spectral information and storage
| SYTO 59 | |
|---|---|
| Excitation/Emission (nm) | 622/645 |
| Standard filter set | Cy®3.5 |
| EVOS Light Cube | Texas Red |
| Storage conditions | ≤–20°C |
Protocol tips
- Warm to room temperature and briefly centrifuge the DMSO solution to the bottom of the vial each time before use.
- Try multiple dye concentrations in the range from 100 nM to 5 µM to determine the optimal concentration.
- In general, the best results are obtained in buffers that do not contain phosphate, such as Hank’s Balanced Salt Solution (14025092).
- Treat all nucleic acid binding dyes as potential mutagens and handle with care.
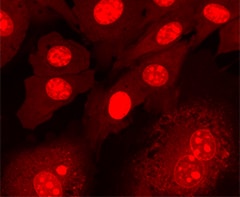
Nuclear staining of BPAECs. BPAECs were cultured, stained with SYTO 59 dye (5 μM for 5 min), and then imaged.